추천 제품
Quality Level
분석
≥98%
양식
powder
저장 온도
−20°C
SMILES string
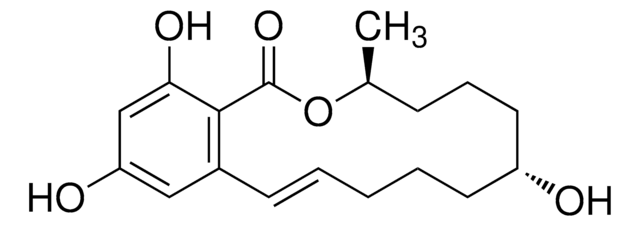
C[C@H]1CCC[C@@H](O)CCC\C=C\c2cc(O)cc(O)c2C(=O)O1
InChI
1S/C18H24O5/c1-12-6-5-9-14(19)8-4-2-3-7-13-10-15(20)11-16(21)17(13)18(22)23-12/h3,7,10-12,14,19-21H,2,4-6,8-9H2,1H3/b7-3+/t12-,14-/m0/s1
InChI key
FPQFYIAXQDXNOR-PMRAARRBSA-N
유전자 정보
rat ... Ar(24208)
애플리케이션
Reactant involved in biological studies including:
- Synthesis of amino glycoside nucleotides
- Enzymatic synthesis of glucuronides of zearalenone
- Derivatization of Zearalenone
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Repr. 2 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Lian Li et al.
Animals : an open access journal from MDPI, 10(1) (2020-01-17)
Zearalenone (ZEA) and T-2 are the most common mycotoxins in grains and can enter the animal and human food-chain and cause many health disorders. To elucidate the toxic response profile, we stimulated bovine granulosa cells (GCs) with β-zearalenol or HT-2.
A Vulić et al.
Journal of analytical toxicology, 43(2), 126-133 (2018-10-09)
Metabolic transformation of zearalenone (ZEA), a mycotoxin which can contaminate both food and feed, results in the formation of five metabolites, one of them being zeranol (α-ZAL), which can be abused in farm animals as a growth promoter. To the
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.