추천 제품
Grade
pharmaceutical primary standard
vapor density
5.24 (vs air)
vapor pressure
4 mmHg ( 70 °C)
API family
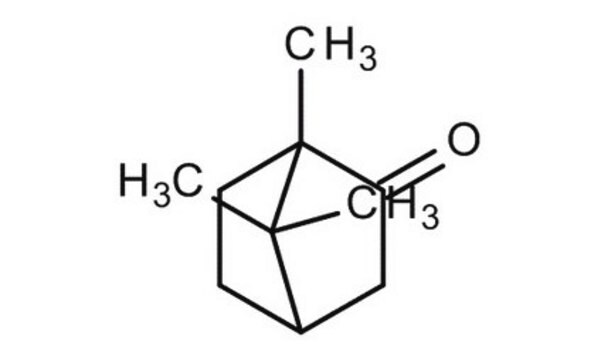
methyl benzylidene camphor
autoignition temp.
870 °F
expl. lim.
3.5 %
제조업체/상표
USP
mp
178-182 °C (lit.)
응용 분야
pharmaceutical (small molecule)
형식
neat
SMILES string
CC1(C)[C@@H]2CC[C@@]1(C)C(=O)C2
InChI
1S/C10H16O/c1-9(2)7-4-5-10(9,3)8(11)6-7/h7H,4-6H2,1-3H3/t7-,10+/m1/s1
InChI key
DSSYKIVIOFKYAU-XCBNKYQSSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Camphor USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Camphorated Phenol Topical Gel
- Camphorated Phenol Topical Solution
- Eucalyptus Oil
분석 메모
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
기타 정보
Sales restrictions may apply.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Eye Dam. 1 - Flam. Sol. 2 - Skin Irrit. 2 - STOT SE 2 Inhalation
표적 기관
Lungs
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 1
Flash Point (°F)
147.2 °F - closed cup
Flash Point (°C)
64 °C - closed cup
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Gustav Georg Belz et al.
Phytomedicine : international journal of phytotherapy and phytopharmacology, 10 Suppl 4, 61-67 (2003-06-17)
Independent, double-blinded, randomized, placebo-controlled studies using sublingual/oral administration of D-camphor, an extract from fresh crataegus berries, and a combination of the two (CCC) yielded the following results: Both the D-camphor and the extract from fresh crataegus berries, the components of
Jeffrey N Love et al.
The Journal of emergency medicine, 27(1), 49-54 (2004-06-29)
Serious pediatric toxicity resulting from exposure to small amounts of camphor-containing products has long been a problem. Twenty years ago the United States Food and Drug Administration took several actions in an attempt to ameliorate this risk. Despite these changes
E Siegel et al.
Pediatric clinics of North America, 33(2), 375-379 (1986-04-01)
Camphor is present in several over-the-counter compounds of questionable use and therefore may be ingested by small children. Because seizures may follow ingestion of certain amounts, appropriate treatment is needed, including the use of anticonvulsants.
Ju-Young Kim et al.
Drug delivery, 21(7), 519-529 (2013-11-20)
The aims of the present study were to prepare new dual-mode floating gastroretentive tablets (DF-GRT) containing itraconazole (ITR) and to evaluate influence of the dosage forms on pharmacokinetic parameters of ITR. The solubility of ITR was enhanced around 200 times
D E Gibson et al.
The American journal of emergency medicine, 7(1), 41-43 (1989-01-01)
Camphor ingestion is a toxic ingestion that is seen infrequently in the emergency department. It is remarkable for its rapidity of action and toxicity. A case of camphor ingestion that displayed toxic effects is presented. The pharmacology, manifestations, and management
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.