116122
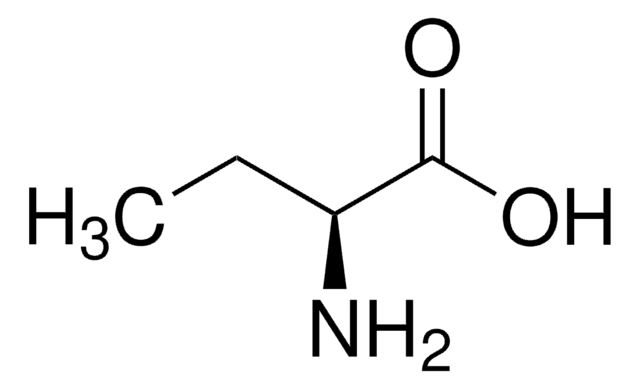
D-2-Aminobutyric acid
98%
Synonym(s):
(R)-(−)-2-Aminobutyric acid
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(3)
Select a Size
Change View
About This Item
Linear Formula:
C2H5CH(NH2)CO2H
CAS Number:
Molecular Weight:
103.12
Beilstein/REAXYS Number:
1720934
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
form
solid
optical activity
[α]20/D −21 to −19°, c = 4 in H2O
optical purity
ee: 98% (GLC)
reaction suitability
reaction type: solution phase peptide synthesis
mp
>300 °C (lit.)
application(s)
peptide synthesis
SMILES string
CC[C@@H](N)C(O)=O
InChI
1S/C4H9NO2/c1-2-3(5)4(6)7/h3H,2,5H2,1H3,(H,6,7)/t3-/m1/s1
InChI key
QWCKQJZIFLGMSD-GSVOUGTGSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chen Qi et al.
Polymers, 12(9) (2020-09-13)
Eight kinds of chiral diacid monomers were prepared with amino acids with different side groups or configurations. Polyester-imides (PEIs) were synthesized from these diacid monomers and diphenol monomers through polycondensation reaction, and the performances and properties were compared with the
Quantification of aminobutyric acids and their clinical applications as biomarkers for osteoporosis.
Zhiying Wang et al.
Communications biology, 3(1), 39-39 (2020-01-24)
Osteoporosis is a highly prevalent chronic aging-related disease that frequently is only detected after fracture. We hypothesized that aminobutyric acids could serve as biomarkers for osteoporosis. We developed a quick, accurate, and sensitive screening method for aminobutyric acid isomers and
Cornelia Reimmann et al.
Journal of bacteriology, 186(19), 6367-6373 (2004-09-18)
In Pseudomonas aeruginosa, the antibiotic dihydroaeruginoate (Dha) and the siderophore pyochelin are produced from salicylate and cysteine by a thiotemplate mechanism involving the peptide synthetases PchE and PchF. A thioesterase encoded by the pchC gene was found to be necessary
Francesco Gasparrini et al.
Journal of the American Chemical Society, 130(2), 522-534 (2007-12-22)
The structure, stability, and reactivity of proton-bound diastereomeric [M x H x A]+ complexes between some amino acid derivatives (A) and several chiral tetra-amide macrocycles (M) have been investigated in the gas phase by ESI-FT-ICR and ESI-ITMS-CID mass spectrometry. The
Napoleon Waszkiewicz et al.
Psychiatria polska, 44(1), 137-146 (2010-05-11)
An increasing number of new biomarkers of alcohol abuse appear in the literature. The most commonly used biomarkers (5-hydroxytryptophol, fatty acid ethyl esters, ethyl glucuronide, phosphatidyl ethanol, ethyl sulphate, mitochondrial aspartate aminotransferase, carbohydrate deficient transferrin, acetaldehyde adducts, beta-hexosaminidase, and sialic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service