162663
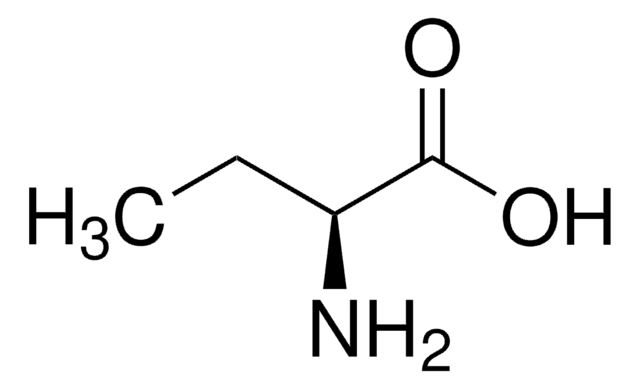
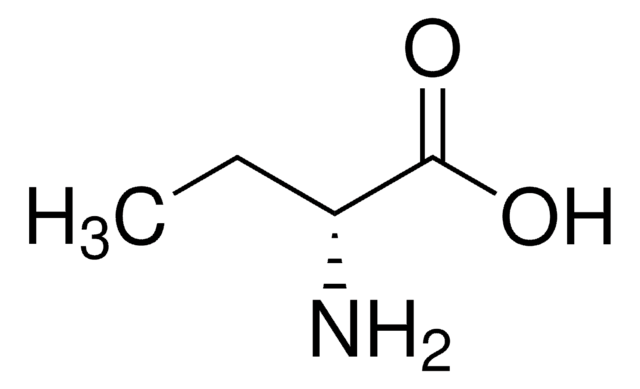
DL-2-Aminobutyric acid
99%, for peptide synthesis, ReagentPlus®
Synonym(s):
AABA, Homoalanine, alpha-amino-n-butyric acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C2H5CH(NH2)CO2H
CAS Number:
Molecular Weight:
103.12
Beilstein/REAXYS Number:
635889
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Product Name
DL-2-Aminobutyric acid, ReagentPlus®, 99%
product line
ReagentPlus®
assay
99%
form
solid
reaction suitability
reaction type: solution phase peptide synthesis
mp
291 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
CCC(N)C(O)=O
InChI
1S/C4H9NO2/c1-2-3(5)4(6)7/h3H,2,5H2,1H3,(H,6,7)
InChI key
QWCKQJZIFLGMSD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
DL-2-Aminobutyric acid also known as α-aminobutyric acid, is commonly used in solution-phase peptide synthesis.
Application
DL-2-Aminobutyric acid can be used to synthesize 2-(2,5-dioxopyrrolidin-1-yl) butanoic acid.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Design, synthesis, and anticonvulsant activity of new hybrid compounds derived from 2-(2, 5-dioxopyrrolidin-1-yl) propanamides and 2-(2, 5-dioxopyrrolidin-1-yl) butanamides
Journal of Medicinal Chemistry, 58, 5274-5286 (2015)
Ryan J Martinie et al.
Journal of the American Chemical Society, 137(21), 6912-6919 (2015-05-13)
The iron(II)- and 2-(oxo)glutarate-dependent (Fe/2OG) oxygenases catalyze an array of challenging transformations, but how individual members of the enzyme family direct different outcomes is poorly understood. The Fe/2OG halogenase, SyrB2, chlorinates C4 of its native substrate, l-threonine appended to the
Danica P Galonić et al.
Nature chemical biology, 3(2), 113-116 (2007-01-16)
Enzymatic incorporation of a halogen atom is a common feature in the biosyntheses of more than 4,500 natural products. Halogenation of unactivated carbon centers in the biosyntheses of several compounds of nonribosomal peptide origin is carried out by a class
Izumi Kawabata et al.
Nature communications, 3, 722-722 (2012-03-08)
Synaptic remodelling coordinated with dendritic growth is essential for proper development of neural connections. After establishment of synaptic contacts, synaptic junctions are thought to become stationary and provide fixed anchoring points for further dendritic growth. However, the possibility of active
Young-Man Seo et al.
Organic & biomolecular chemistry, 10(12), 2482-2485 (2012-02-22)
A deracemization method was developed to generate optically pure L-homoalanine from racemic homoalanine using D-amino acid oxidase and ω-transaminase. A whole cell reaction using a biphasic system converted 500 mM racemic homoalanine to 485 mM L-homoalanine (>99% ee).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service