121207
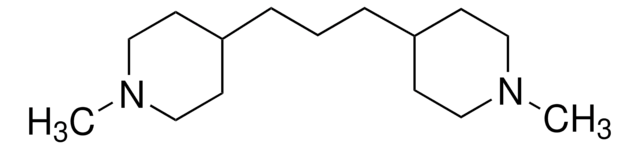
4,4′-Trimethylenedipiperidine
97%
Synonym(s):
1,3-Bis(4-piperidyl)propane, 1,3-Di-4-piperidylpropane
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C13H26N2
CAS Number:
Molecular Weight:
210.36
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
mp
65-68.5 °C (lit.)
SMILES string
C(CC1CCNCC1)CC2CCNCC2
InChI
1S/C13H26N2/c1(2-12-4-8-14-9-5-12)3-13-6-10-15-11-7-13/h12-15H,1-11H2
InChI key
OXEZLYIDQPBCBB-UHFFFAOYSA-N
General description
4,4′-Trimethylenedipyridine undergoes Willgerodt-Kindler reaction by on pot- polycondensation with dialdehydes in the presence of sulphur to form polythioamides.
Application
4,4′-Trimethylenedipiperidine was used in the synthesis of hyperbranched copolymers from commercially available monomers: divinylsulfone, 4,4′-trimethylenedipiperidine and N-ethylenediamine. It was used in the synthesis of two- and three-dimensional cadmium-organic frameworks.
Reactant for synthesis of:
- Tumoral pH-responsive polymeric micelles
- Acetal modified dextran microparticles
- Hydrogels
- Functionalized tetrazoles
- Anticonvulsant agents
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hyperbranched copolymers made from A2, B2 and BB' 2type monomers (iv).
Gao C, et al.
Science in China. Series B, Chemistry, Life Sciences & Earth Sciences, 44(2), 207-215 (2001)
Stefano Perni et al.
Nanomedicine : nanotechnology, biology, and medicine, 13(2), 539-548 (2016-10-27)
The efficient delivery of therapeutic molecules to the cartilage of joints is a major obstacle in developing useful therapeutic interventions; hence, a targeted drug delivery system for this tissue is critical. We have overcome the challenge by developing a system
Preparation of polythioamides from dialdehydes and 4, 4'-trimethylenedipiperidine with sulfur by the Willgerodt-Kindler reaction.
Kawai Y, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 37(12), 1737-1740 (1999)
Filipe A Almeida Paz et al.
Inorganic chemistry, 43(13), 3882-3893 (2004-06-23)
Three novel cadmium-organic frameworks built-up from 1,3,5-benzenetricarboxylate anions (HXBTC(x-3)) and 4,4'-trimethylenedipyridine (TMD) have been hydrothermally synthesized, and characterized using single-crystal X-ray diffraction, thermoanalytical measurements, elemental analysis, and IR and Raman spectroscopies: [Cd(HBTC)(TMD)(2)].8.5H(2)O (I), [Cd(HBTC)(TMD)(H(2)O)].4.5H(2)O (II), and [Cd(2)(BTC)(TMD)(2)(NO(3))].3H(2)O (III), with structures
Weinan Li et al.
Pharmaceutics, 12(2) (2020-02-06)
PEG-poly(β-amino ester) (PEG-PBAE), which is an effective pH-responsive copolymer, was mainly synthesized by Michael step polymerization. Thioridazine (Thz), which was reported to selectively eliminate cancer stem cells (CSCs), was loaded into PEG-PBAE micelles (PPM) prepared by self-assembly at low concentrations.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service