122513
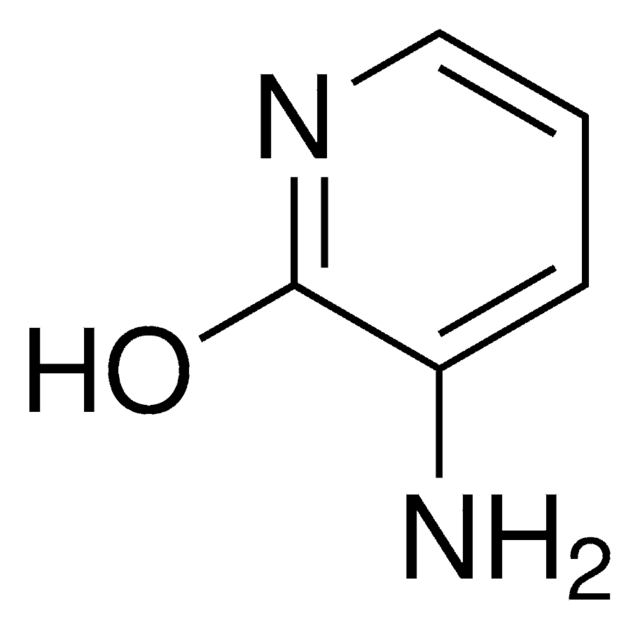
2-Amino-3-hydroxypyridine
98%
Synonym(s):
2-Amino-3-pyridinol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H6N2O
CAS Number:
Molecular Weight:
110.11
Beilstein/REAXYS Number:
109868
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39151903
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
mp
168-172 °C (lit.)
SMILES string
Nc1ncccc1O
InChI
1S/C5H6N2O/c6-5-4(8)2-1-3-7-5/h1-3,8H,(H2,6,7)
InChI key
BMTSZVZQNMNPCT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Amino-3-hydroxypyridine forms complexes with number of transition metals. It inhibits the corrosion of aluminium and copper in acidic solutions. It undergoes condensation with 2-hydroxy-1-naphthaldehyde and 2-hydroxybenzaldehyde to form Schiffs bases. It is useful in the preparation of clinical anti-inflammatory analgesics.
Application
2-Amino-3-hydroxypyridine was used as reagent in reaction of dimethyl acetylenedicarboxylate and triphenylphosphine to yield functionalized coumarins and 1,4-oxazines.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and characterization of some transition metal complexes of 2-amino-3-hydroxypyridine and its application in corrosion inhibition.
Mostafa SI and El-Maksoud SA.
Monatshefte fur Chemie / Chemical Monthly, 129(5), 455-466 (1998)
1 H NMR, IR and UV/VIS Spectroscopic Studies of Some Schiff Bases Derived From 2-Aminobenzothiazole and 2-Amino-3-hydroxypyridine.
Issa RM, et al.
J. Chin. Chem. Soc., 55(4), 875-884 (2008)
Vinyltriphenylphosphonium salt mediated synthesis of 1, 4-benzoxazine and coumarin derivatives.
Yavari I, et al.
Tetrahedron, 58(34), 6895-6899 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service