126268
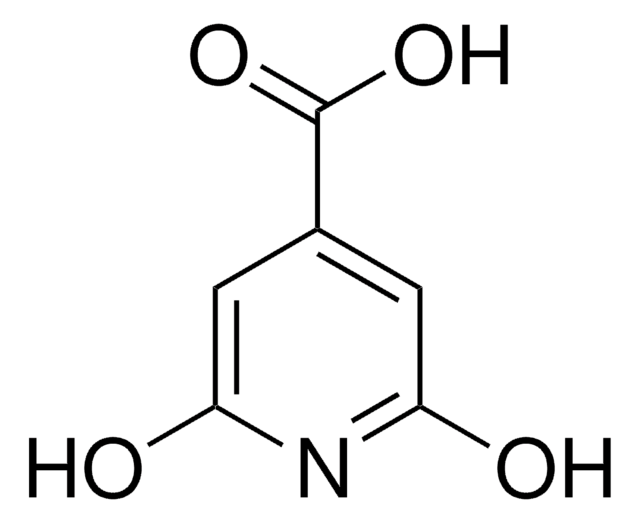
2,4-Dihydroxypyrimidine-5-carboxylic acid
95%
Synonym(s):
Isoorotic acid, Uracil-5-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H4N2O4
CAS Number:
Molecular Weight:
156.10
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
95%
form
powder
mp
283 °C (dec.) (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)C1=CNC(=O)NC1=O
InChI
1S/C5H4N2O4/c8-3-2(4(9)10)1-6-5(11)7-3/h1H,(H,9,10)(H2,6,7,8,11)
InChI key
ZXYAAVBXHKCJJB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
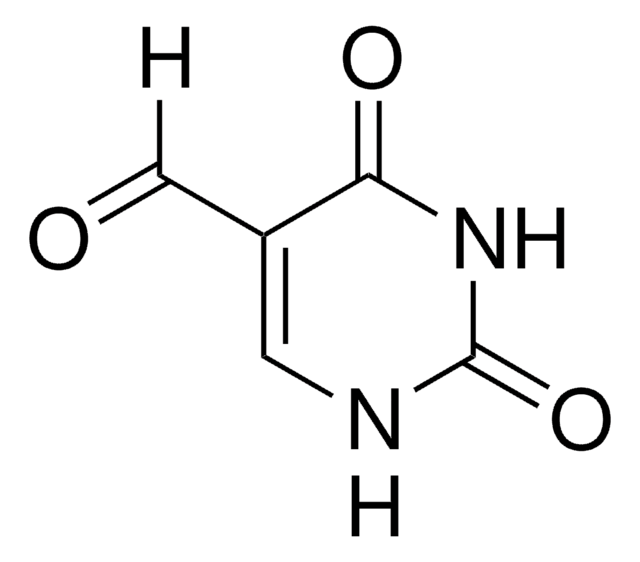
2,4-Dihydroxypyrimidine-5-carboxylic acid (Uracil-5-carboxylic acid) has been obtained from 5-formyluracil by the action of enzyme, thymine 7-hydroxylase. It has been used to synthesize N1-alkylated uracil derivatives.
Application
2,4-Dihydroxypyrimidine-5-carboxylic acid (Uracil-5-carboxylic acid) has been used for the visual sensing of melamine (at parts-per-billion (ppb) level) by a highly sensitive analytical method based on Au nanoparticles.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P M Shaffer et al.
Journal of bacteriology, 121(2), 648-655 (1975-02-01)
The experiments in this report involve the following series of reactions which were previously demonstrated with purified enzyme preparations from Neurospora crassa: thymidine a yields thymine ribonucleoside b yields thymine c yields 5-hydroxymethyluracil d yields 5-formyluracil e yields uracil-5-carboxylic acid
Raj Kumar Bera et al.
The Analyst, 136(8), 1644-1648 (2011-02-25)
A highly sensitive analytical method based on Au nanoparticles rationally tailored with recognition elements uracil-5-carboxylic acid (UCA) and 2,4,6-trinitrobenzenesulfonic acid (TNBS) for the visual sensing of melamine at the parts-per-billion (ppb) level is described. The tailored Au nanoparticles function as
Alessandro Accetta et al.
Journal of medicinal chemistry, 52(1), 87-94 (2008-12-17)
The synthesis of C5 linked uracil dimers was carried out according to a model developed in order to bind adenine in DNA. N1-Alkylated uracil derivatives were synthesized from isoorotic acid (uracil-5-carboxylic acid) or thymine. The carboxylic acid derivatives were condensed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service