All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H9N3O2
CAS Number:
Molecular Weight:
155.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
mp
149-152 °C (lit.)
solubility
methanol: soluble 25 mg/mL, clear, colorless to yellow
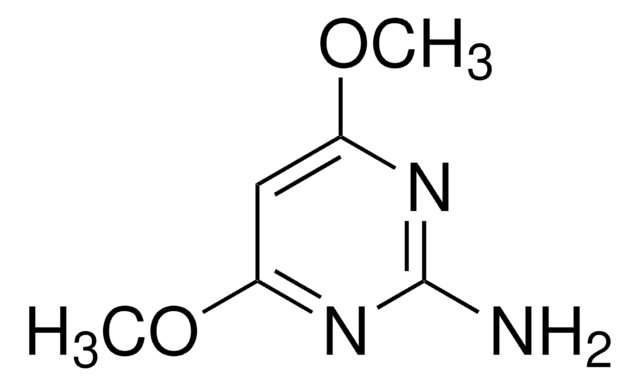
SMILES string
COc1cc(N)nc(OC)n1
InChI
1S/C6H9N3O2/c1-10-5-3-4(7)8-6(9-5)11-2/h3H,1-2H3,(H2,7,8,9)
InChI key
LNTJJKHTAZFVJJ-UHFFFAOYSA-N
Related Categories
General description
4-Amino-2,6-dimethoxypyrimidine is methoxy substituted 4-aminopyrimidine. Molecules of 4-amino-2,6-dimethoxypyrimidine are linked by an N-H.O hydrogen bond and an N-H.N hydrogen bond, forming sheets containing centrosymmetric rings. Photocatalytic degradation of 4-amino-2,6-dimethoxypyrimidine on TiO2 has been reported. Mass spectra of 4-amino-2,6-dimethoxypyrimidine has been studied.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mass spectra of methoxy-substituted 4-aminopyrimidines.
Khmel'nitskii RA, et al.
Chemistry of Heterocyclic Compounds, 10(1), 113-116 (1974)
Christopher Glidewell et al.
Acta crystallographica. Section C, Crystal structure communications, 59(Pt 4), O202-O204 (2003-04-12)
Molecules of the title compound, C(6)H(9)N(3)O(2), are linked by an N-H.O hydrogen bond [H.O = 2.29 A, N.O = 3.169 (2) A and N-H.O = 173 degrees ] and an N-H.N hydrogen bond [H.N = 2.12 A, N.N = 2.999
Photocatalytic transformations of aminopyrimidines on TiO< sub> 2</sub> in aqueous solution.
Calza P, et al.
Applied Catalysis. B, Environmental, 52(4), 267-274 (2004)
Monica Olivella et al.
Archiv der Pharmazie, 348(1), 68-80 (2014-11-22)
New nitrosopyrimidines were synthesized and evaluated as potential antibacterial agents. Different compounds structurally related with 4,6-bis(alkyl or arylamino)-5-nitrosopyrimidines were evaluated. Some of these nitrosopyrimidines displayed significant antibacterial activity against human pathogenic bacteria. Among them compounds 1c, 2a-c, and 9a-c exhibited
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service