140392
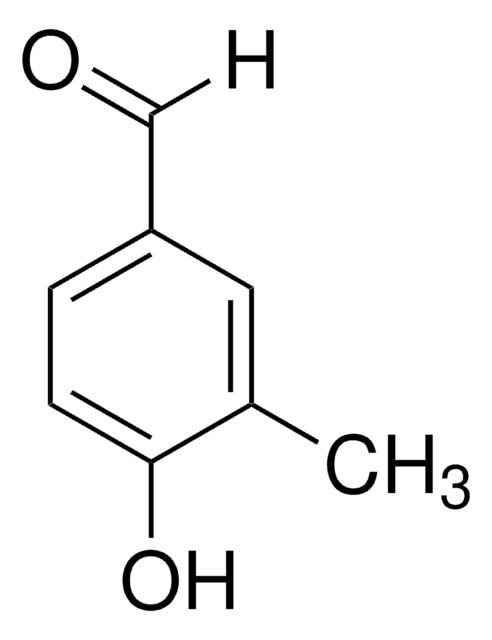
4-Hydroxy-3,5-dimethylbenzaldehyde
95%
Synonym(s):
3,5-Dimethyl-4-hydroxybenzaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HOC6H2(CH3)2CHO
CAS Number:
Molecular Weight:
150.17
Beilstein/REAXYS Number:
1908717
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
95%
mp
112-114 °C (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)c1cc(C)c(O)c(C)c1
InChI
1S/C9H10O2/c1-6-3-8(5-10)4-7(2)9(6)11/h3-5,11H,1-2H3
InChI key
UYGBSRJODQHNLQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Hydroxy-3,5-dimethylbenzaldehydewas used as reagent during the phase transfer catalyzed polymerization of 4-hydroxy-3,5-dimethylbenzyl alcohol. It was used as starting reagent during the synthesis of 2,4,6-trimethylphenol.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Phase transfer catalyzed polymerization of 4-hydroxy-3, 5-dimethylbenzyl alcohol and copolymerization of 4-bromo-2, 6-dimethylphenol with 4-hydroxy-3, 5-dimethylbenzyl alcohol.
Wang JH and Percec V.
Polym. Bull., 25(1), 25-32 (1991)
D J Abraham et al.
Biochemistry, 34(46), 15006-15020 (1995-11-21)
Monoaldehyde allosteric effectors of hemoglobin were designed, using molecular modeling software (GRID), to form a Schiff base adduct with the Val 1 alpha N-terminal nitrogens and interact via a salt bridge with Arg 141 alpha of the opposite subunit. The
A facile synthesis of 4-alkoxymethylphenols by a copper (II)-acetoxime catalyst/O2 system.
Shimizu M, et al.
Tetrahedron Letters, 32(18), 2053-2056 (1991)
P R Ortiz de Montellano et al.
The Journal of biological chemistry, 262(24), 11641-11646 (1987-08-25)
Chloroperoxidase and H2O2 oxidize styrene to styrene oxide and phenylacetaldehyde but not benzaldehyde. The epoxide oxygen is shown by studies with H2(18)O2 to derive quantitatively from the peroxide. The epoxidation of trans-[1-2H]styrene by chloroperoxidase proceeds without detectable loss of stereochemistry
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service