177199
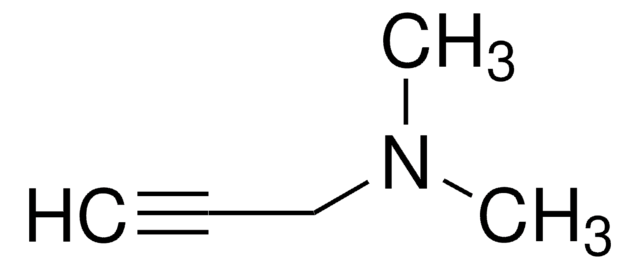
Methyl propargyl ether
≥97%
Synonym(s):
3-Methoxy-1-propyne, Methyl 2-propynyl ether
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HC≡CCH2OCH3
CAS Number:
Molecular Weight:
70.09
Beilstein/REAXYS Number:
878166
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥97%
form
liquid
refractive index
n20/D 1.396 (lit.)
bp
61-62 °C (lit.)
density
0.83 g/mL at 25 °C (lit.)
functional group
ether
storage temp.
2-8°C
SMILES string
COCC#C
InChI
1S/C4H6O/c1-3-4-5-2/h1H,4H2,2H3
InChI key
YACFFSVYSPMSGS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The Raman spectra of methyl propargyl ether has been studied.
Application
Methyl propargyl ether was used in the synthesis of [Ru3(μ3-κ(2)-HNNMe2)(μ3-κ(2)-MeOCH2CCH2)(μ-CO)2(CO)6].
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
supp_hazards
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
-0.4 °F - closed cup
flash_point_c
-18 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Javier A Cabeza et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 11(20), 6040-6052 (2005-07-30)
The reactions of the hydrido-triruthenium cluster complex [Ru3(mu-H)(mu3-kappa(2)-HNNMe2)(CO)9] (1; H2NNMe2 = 1,1-dimethylhydrazine) with alkynes that have alpha-hydrogen atoms give trinuclear derivatives containing edge-bridging allyl or face-capping alkenyl ligands. Under mild conditions (THF, 70 degrees C) the isolated products are as
Conformational stability, barriers to internal rotation, ab initio calculations and vibrational assignment of methyl propargyl ether.
Journal of Molecular Structure, 320, 193-216 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service