190357
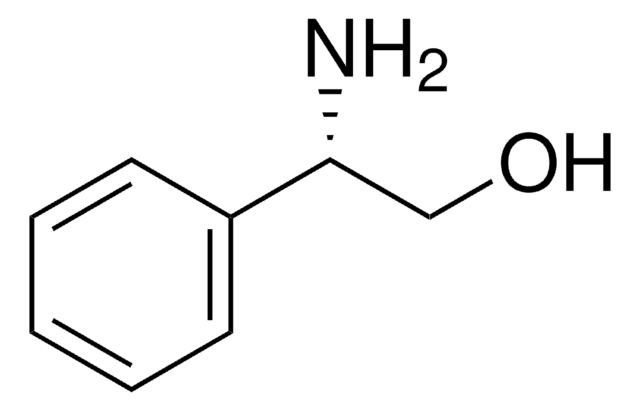
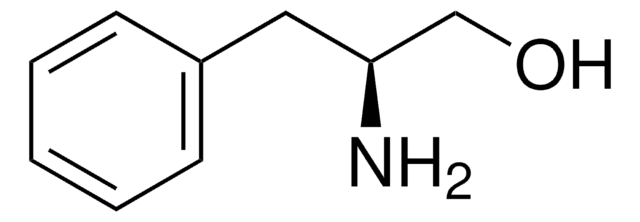
(R)-(−)-2-Phenylglycinol
98%, for peptide synthesis
Synonym(s):
(R)-2-Amino-2-phenylethanol, D-(−)-α-Phenylglycinol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH(NH2)CH2OH
CAS Number:
Molecular Weight:
137.18
Beilstein/REAXYS Number:
2935848
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
product name
(R)-(−)-2-Phenylglycinol, 98%
Quality Level
assay
98%
optical activity
[α]24/D −31.7°, c = 0.76 in 1 M HCl
optical purity
ee: 99% (GLC)
reaction suitability
reaction type: solution phase peptide synthesis
mp
75-77 °C (lit.)
application(s)
peptide synthesis
SMILES string
N[C@@H](CO)c1ccccc1
InChI
1S/C8H11NO/c9-8(6-10)7-4-2-1-3-5-7/h1-5,8,10H,6,9H2/t8-/m0/s1
InChI key
IJXJGQCXFSSHNL-QMMMGPOBSA-N
Application
Amino alcohol used to prepare a chiral imine or oxazolidine from ethyl trifluoropyruvate. These intermediates were then employed in a synthesis of both enantiomers of α-trifluoromethylproline.
Chiral β−amino alcohol used as a synthetic building block.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Grégory Chaume et al.
Organic letters, 8(26), 6123-6126 (2006-12-15)
[Structure: see text] A concise synthesis of both enantiomers of alpha-Tfm-proline and (S)-alpha-Tfm-prolinol from ethyl trifluoropyruvate is reported. The key step is a diastereoselective allylation reaction of ethyl trifluoropyruvate and (R)-phenylglycinol-based oxazolidines or imine. The lactone obtained by cyclization of
Tetrahedron Letters, 45, 5287-5287 (2004)
Amedjkouh, M.; Westerlund, K.
Tetrahedron Letters, 45, 5175-5175 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service