191558
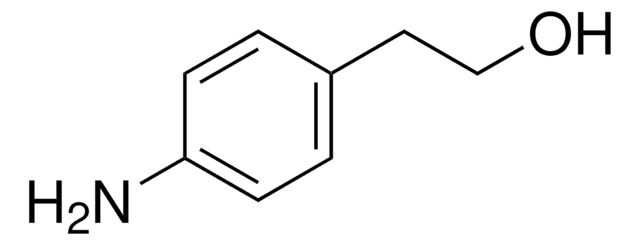
4-Aminobenzyl alcohol
98%
Synonym(s):
4-(Hydroxymethyl)aniline
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
H2NC6H4CH2OH
CAS Number:
Molecular Weight:
123.15
Beilstein/REAXYS Number:
2078680
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
mp
60-65 °C (lit.)
functional group
hydroxyl
storage temp.
2-8°C
SMILES string
Nc1ccc(CO)cc1
InChI
1S/C7H9NO/c8-7-3-1-6(5-9)2-4-7/h1-4,9H,5,8H2
InChI key
AXKGIPZJYUNAIW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Aminobenzyl alcohol is used as a starting material to synthesize other organic compounds.
Application
4-Aminobenzyl alcohol can be used:
- In the synthesis of 4-{N-[1-(4,4-dimethyl-2,6-dioxocyclohexylidene)-3-methylbutylamino}benzyl ester.
- As a reactant to synthesize cross-azo compounds and cathepsin B cleavable dipeptide linker.
- As a starting material to synthesize hydrogelators for drug delivery applications.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Selective Single-Step Oxidation of Amine to Cross-Azo Compounds with an Unhampered Primary Benzyl Alcohol Functionality
Sarkar S, et al.
Organic Letters, 20(21), 6725-6729 (2018)
Supramolecular hydrogels for enzymatically triggered self-immolative drug delivery
Saez J, et al.
Tetrahedron, 66(14), 2614-2618 (2010)
Improved Methodology for the Synthesis of a Cathepsin B Cleavable Dipeptide Linker, Widely Used in Antibody-Drug Conjugate Research
Mondal D, et al.
Tetrahedron Letters, 59(40), 3594-3599 (2018)
Katarzyna Gorska et al.
Chemical communications (Cambridge, England), 47(15), 4364-4366 (2011-03-04)
Nucleic acid templated reactions have attracted significant attention for nucleic acid sensing. Herein we report a general design which extends the potential of nucleic acid templated reactions to unleash the function of a broad diversity of small molecules such as
The rational design of a peptide-based hydrogel responsive to H 2 S
Peltier R, et al.
Chemical Communications (Cambridge, England), 51(97), 17273-17276 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service