All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H8N2
CAS Number:
Molecular Weight:
108.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
solid
bp
100 °C/0.1 mmHg (lit.)
mp
124-125 °C (lit.)
functional group
amine
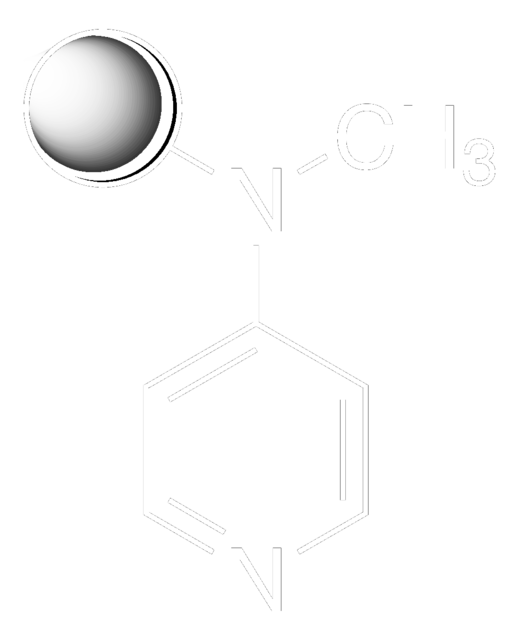
SMILES string
CNc1ccncc1
InChI
1S/C6H8N2/c1-7-6-2-4-8-5-3-6/h2-5H,1H3,(H,7,8)
InChI key
LSCYTCMNCWMCQE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-(Methylamino)pyridine can be used:
- To functionalize hypercrosslinked emulsion-templated porous polymers (polyHIPE) to form a highly efficient heterogeneous nucleophilic catalyst for the acylation of a tertiary alcohol.
- As a reactant to synthesize 4-(N-allyl-N-methylamino)pyridine, which is employed as an intermediate to prepare DMAP/SBA-15 supported catalyst for the synthesis of propylene carbonate.
- To prepare 5-azaoxindoles via homolytic aromatic substitution.
4-(Methylamino)pyridine was employed as efficient nucleophilic catalyst during the preparation of ultra-high surface area emulsion templated porous polymers.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Homolytic aromatic substitution: A radical approach towards the synthesis of 5-azaoxindoles
Storey J MD and Ladwa MM
Tetrahedron Letters, 47(3), 381-383 (2006)
Homogeneous and heterogeneous 4-(N, N-dialkylamino) pyridines as effective single component catalysts in the synthesis of propylene carbonate
Shiels RA and Jones CW
J. Mol. Catal. A: Chem., 261(2), 160-166 (2007)
Ultra-high surface area functional porous polymers by emulsion templating and hypercrosslinking: efficient nucleophilic catalyst supports
Pulko I, et al.
Chemistry?A European Journal , 16(8), 2350-2354 (2010)
Y Amaki et al.
Masui. The Japanese journal of anesthesiology, 38(5), 661-665 (1989-05-01)
A new muscle relaxant antagonist, 4-aminopyridine (4-AMP), has various problems related to its side effects. In order to obtain 4-AMP derivatives with less side effect and the same antagonizing effect on dTc block as that of 4-AMP, three types of
Ultra-high surface area functional porous polymers by emulsion templating and hypercrosslinking: efficient nucleophilic catalyst supports.
Irena Pulko et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 16(8), 2350-2354 (2010-01-28)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service