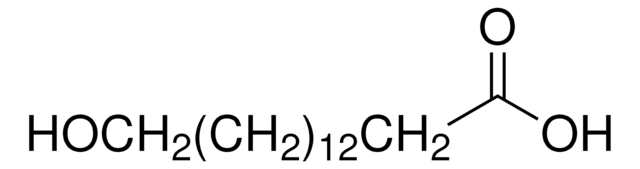198781
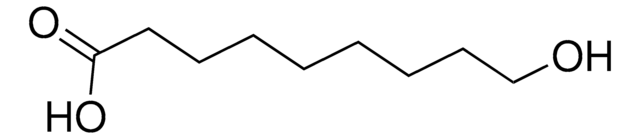
12-Hydroxydodecanoic acid
97%
Synonym(s):
12-Hydroxylauric acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
HO(CH2)11COOH
CAS Number:
Molecular Weight:
216.32
Beilstein/REAXYS Number:
1238370
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
mp
85-88 °C (lit.)
functional group
carboxylic acid
hydroxyl
SMILES string
OCCCCCCCCCCCC(O)=O
InChI
1S/C12H24O3/c13-11-9-7-5-3-1-2-4-6-8-10-12(14)15/h13H,1-11H2,(H,14,15)
InChI key
ZDHCZVWCTKTBRY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
12-Hydroxydodecanoic acid was used in the synthesis of high molecular weight poly[(12-hydroxydodecanoate)-co-(12-hydroxystearate)] [poly(12HD-co-12HS)] samples with variable monomer ratios using methyl 12-hydroxystearate.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Alexander N Grechkin et al.
FEBS letters, 549(1-3), 31-34 (2003-08-14)
Guava (Psidium guajava) hydroperoxide lyase (HPL) preparations were incubated with [1-(14)C](9Z,11E,13S,15Z)-13-hydroperoxy-9,11,15-octadecatrienoic acid for 1 min at 0 degrees C, followed by rapid extraction/trimethylsilylation. Analysis of the trimethylsilylated products by gas chromatography-mass spectrometry and radio-high-performance liquid chromatography revealed a single predominant
Elena Bailo et al.
Analytical and bioanalytical chemistry, 394(7), 1797-1801 (2009-06-16)
Surface-enhanced Raman scattering was used as a spectroscopic tool to investigate the changes brought upon cytochrome P450BSss after fatty acid binding. Differences in the spectra of substrate-free and substrate-bound enzyme were observed indicating the potential for this method to be
J J Hedberg et al.
FEBS letters, 436(1), 67-70 (1998-10-15)
Human class I alcohol dehydrogenase was mutated at positions 57 and 115, exchanging for Asp and Arg respectively, in an attempt to introduce glutathione-dependent formaldehyde dehydrogenase characteristics. In addition, class III alcohol dehydrogenase, identical to glutathione-dependent formaldehyde dehydrogenase, was mutated
D D Giera et al.
Fundamental and applied toxicology : official journal of the Society of Toxicology, 16(2), 348-355 (1991-02-01)
Assessment of hepatic omega-oxidation of fatty acids by cytochrome P450IV enzymes in toxicology studies can be a means of evaluating test compound effects on peroxisomal proliferation. Routine assay of omega-oxidation, however, requires a simpler method of enzymatic analysis than currently
H A Dirven et al.
Journal of chromatography, 564(1), 266-271 (1991-03-08)
The formation of omega-hydroxylauric acid from lauric acid is an indicator of the activity of cytochrome P-450 IV family proteins. The two main metabolites of lauric acid, (omega-1)-and omega-hydroxylauric acid, have been completely separated by reversed-phase high-performance liquid chromatography. Measurement
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service