All Photos(1)
About This Item
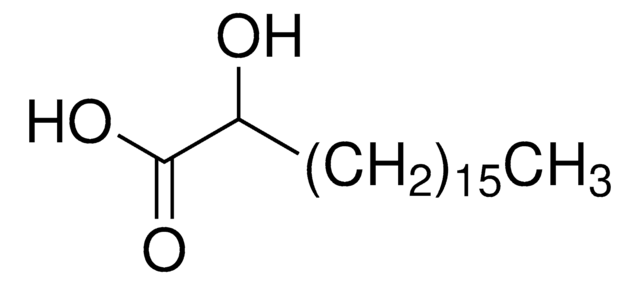
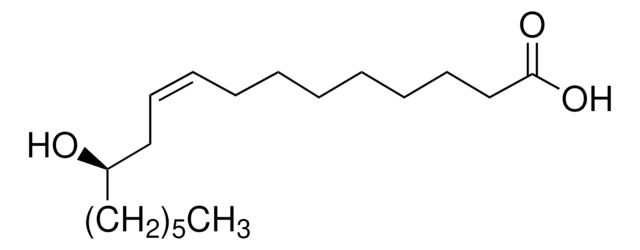
Linear Formula:
HO(CH2)14CO2H
CAS Number:
Molecular Weight:
258.40
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
mp
85-89 °C (lit.)
functional group
carboxylic acid
hydroxyl
SMILES string
OCCCCCCCCCCCCCCC(O)=O
InChI
1S/C15H30O3/c16-14-12-10-8-6-4-2-1-3-5-7-9-11-13-15(17)18/h16H,1-14H2,(H,17,18)
InChI key
BZUNJUAMQZRJIP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
15-Hydroxypentadecanoic acid is an ω-hydroxy acid. One of the method reported for its synthesis is from 1,12-dodecanolide. It is reported to be one of the bioactive component in Tagetes erecta L. leaf and flower extract.
15-Hydroxypentadecanoic acid undergoes lactonization reaction catalyzed by Mucor javanicus L46 and Mucor miehei to afford macrocyclic mono- and oligolactone derivatives. Its lipase-catalyzed synthesis from 15-tetracosenoic acid in Malania Olcifera Chum oil has been proposed. It also participates in the biosynthesis of pentadecanolide.
Application
15-Hydroxypentadecanoic acid is suitable reagent used in the following studies:
- As an internal standard in the quantification of formation of 11-hydroxylauric acid by gas chromatography.
- In the synthesis of [16-14C]16DCA (DCA= dicarboxylic acid) by one-carbon elongation procedure at C15.
- As an internal standard for the normalization of intensities in the mass spectra of plant cutin polymer.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Preparation of 15-hydroxypentadecanoic acid by means of condensation reaction via β-ketosulfoxide.
Nozaki H, et al.
Canadian Journal of Chemistry, 46(23), 3767-3770 (1968)
Dušan Veličković et al.
The Plant journal : for cell and molecular biology, 80(5), 926-935 (2014-10-04)
The cutin polymers of different fruit cuticles (tomato, apple, nectarine) were examined using matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI MSI) after in situ release of the lipid monomers by alkaline hydrolysis. The mass spectra were acquired from each coordinate
Enzymatic lactonization of 15-hydroxypentadecanoic and 16-hydroxyhexadecanoic acids to macrocyclic lactones.
Antczak U, et al.
Enzyme and Microbial Technology, 13(7), 589-593 (1991)
Zeolite-catalyzed macrolactonization of Ookoshi T and Onaka M. ω-hydroxyalkanoic acids in a highly concentrated solution.
Ookoshi T and Onaka M.
Tetrahedron Letters, 39(3), 293-296 (1998)
Screening and evaluation of bioactive components of Tagetes erecta L. by GC-MS analysis.
Devika R and Kovilpillai J.
Asian Journal of Pharmaceutical and Clinical Research, 7(2), 58-60 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![2-[2-(2-Methoxyethoxy)ethoxy]acetic acid technical grade](/deepweb/assets/sigmaaldrich/product/structures/335/694/b58c539b-141f-4ab2-98d9-5f46c748490b/640/b58c539b-141f-4ab2-98d9-5f46c748490b.png)