All Photos(3)
About This Item
Empirical Formula (Hill Notation):
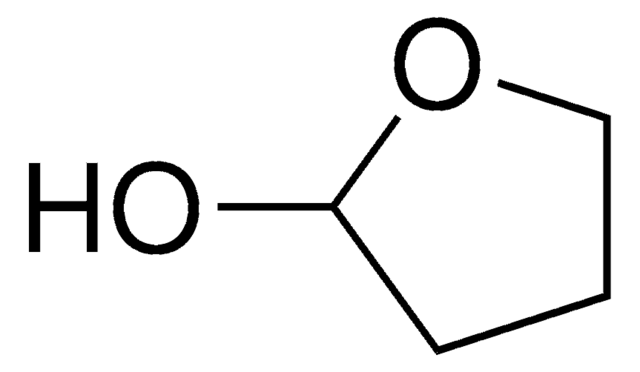
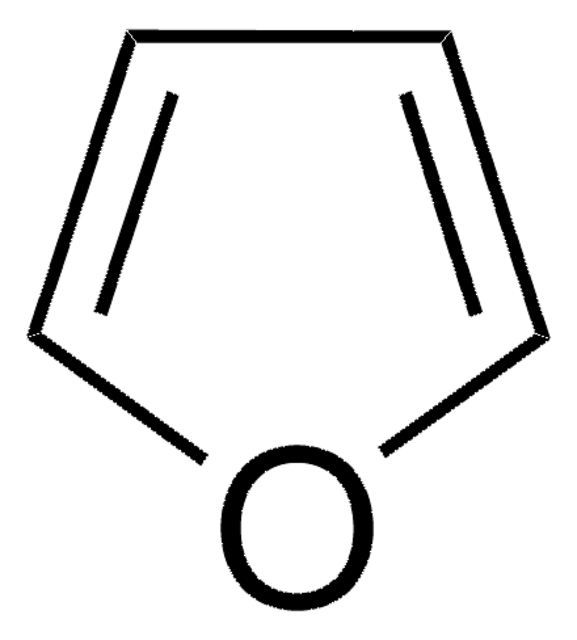
C4H6O
CAS Number:
Molecular Weight:
70.09
Beilstein/REAXYS Number:
103168
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
14.46 psi ( 55 °C)
3.67 psi ( 20 °C)
Quality Level
assay
99%
form
liquid
refractive index
n20/D 1.423 (lit.)
bp
54-55 °C (lit.)
density
0.927 g/mL at 25 °C (lit.)
functional group
ether
Storage temp.
2-8°C
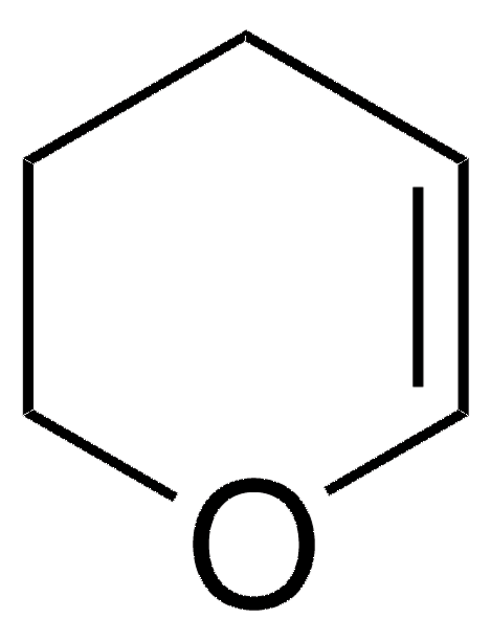
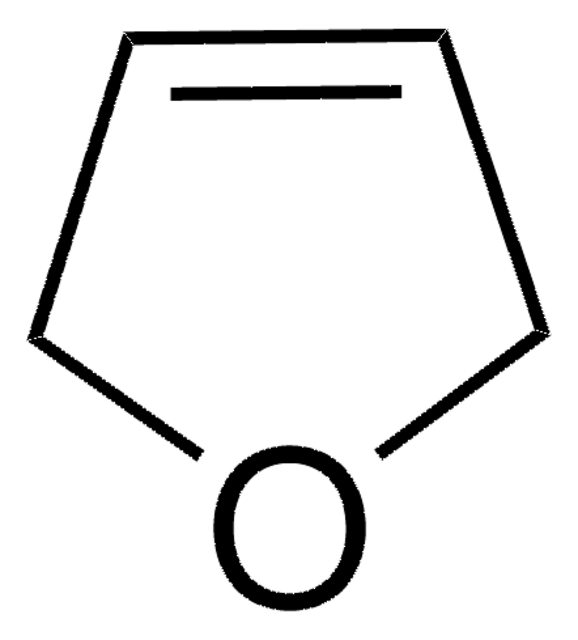
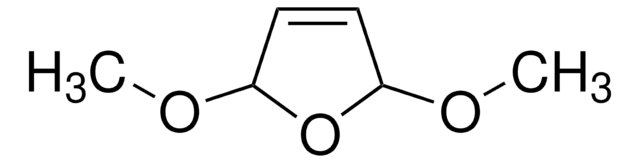
SMILES string
C1CC=CO1
InChI
1S/C4H6O/c1-2-4-5-3-1/h1,3H,2,4H2
Inchi Key
JKTCBAGSMQIFNL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The enantioselective Heck arylation of 2,3-dihydrofuran with aryl iodides was studied.
Application
2,3-Dihydrofuran is a versatile reagent used in lanthanide-catalyzed Diels-Alder reactions with 2-pyrones and in Rh(II)-stabilized cycloadditions with vinylcarbenoids.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
supp_hazards
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
-11.2 °F - closed cup
flash_point_c
-24 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Claudia G Cobo-Angel et al.
Scientific reports, 9(1), 14025-14025 (2019-10-03)
Group B Streptococcus (GBS), is a leading cause of neonatal death and an emerging pathogen in adults. Additionally, GBS is a bovine pathogen causing intramammary infections. The likelihood of GBS interspecies transmission is largely unknown. We explored the potential transmission
Adam Morel et al.
Dalton transactions (Cambridge, England : 2003), 42(4), 1215-1222 (2012-11-09)
Chiral ionic liquids (CILs) containing L-prolinate and L-lactate anions and non-chiral quaternary ammonium cations were employed in the palladium catalyzed enantioselective Heck arylation of 2,3-dihydrofuran with aryl iodides (iodobenzene, 4-iodotoluene, 2-iodoanisole, 4-iodoanisole, 4-iodoacetophenone). In all the reactions 2-aryl-2,3-dihydrofuran (3) was
Qi-Fang Wang et al.
The Journal of organic chemistry, 74(19), 7403-7406 (2009-09-11)
An efficient synthetic procedure for the preparation of fused 2,3-dihydrofuran derivatives was developed with the assistance of pyridinium ylide. A sequential one-pot, two-step tandem reaction starting from pyridine, aromatic aldehyde, dimedone, or 4-hydroxycoumarin and alpha-phenacyl bromide or p-nitrobenzyl bromide with
Tetrahedron, 50, 4557-4557 (1994)
Synlett, 431-431 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service