209058
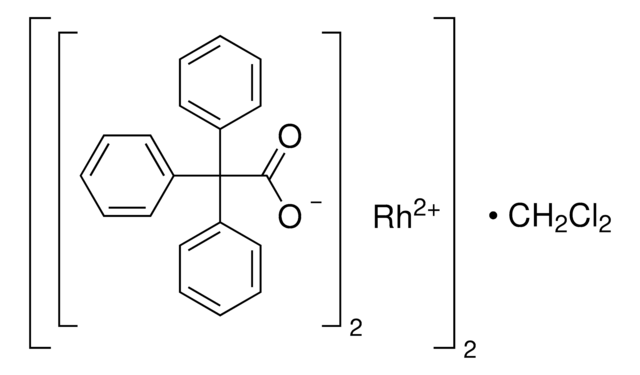
Rhodium(II) acetate dimer
powder
Synonym(s):
Rh2(OAc)4, Dirhodium tetraacetate, Tetrakis(acetato)dirhodium(II)
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(3)
Select a Size
Change View
About This Item
Linear Formula:
Rh2(OOCCH3)4
CAS Number:
Molecular Weight:
441.99
EC Number:
MDL number:
UNSPSC Code:
12161600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
powder
Quality Level
reaction suitability
core: rhodium
reagent type: catalyst
SMILES string
CC(=O)O[Rh]OC(C)=O.CC(=O)O[Rh]OC(C)=O
InChI
1S/4C2H4O2.2Rh/c4*1-2(3)4;;/h4*1H3,(H,3,4);;/q;;;;2*+2/p-4
InChI key
SYBXSZMNKDOUCA-UHFFFAOYSA-J
Looking for similar products? Visit Product Comparison Guide
General description
Rhodium(II) acetate dimer is an effective catalyst for carbenoid reactions, hydrocarbon oxidation, hydroboration, and nitrene reactions.
Application
Effective catalyst for ylide formation.
Homogeneous catalyst.
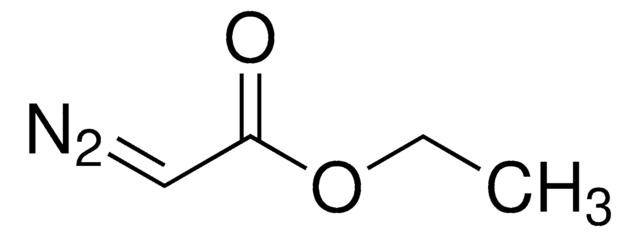
Used in an efficient synthesis of ß-hydroxy-α-arylacrylates involving the decomposition of diazoetster intermediates with concomitant 1,2-arylmigration.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Aldrichimica Acta, 15, 13-13 (1982)
Sugimura, T.; Mori, A.
Tetrahedron Asymmetry, 14, 881-881 (2003)
Dirhodium(II) Tetraacetate
Michael P, et al.
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2001)
Catalytic enantioselective C-H activation by means of metal-carbenoid-induced C-H insertion.
Huw M L Davies et al.
Chemical reviews, 103(8), 2861-2904 (2003-08-14)
Shifa Zhu et al.
Organic letters, 6(3), 377-380 (2004-01-30)
[reaction: see text] Rh(2)(OAc)(4) catalyzed the formation of exclusively trans fluorinated alkenes from aldehydes and pentafluorobenzaldehyde tosylhydrazone salts, which were readily prepared from pentafluorobenzaldehyde using the Bamford-Stevens reaction. A series of pentafluorophenyl-containing alkenes were synthesized from aldehydes in moderate to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Bis[rhodium(α,α,α′,α′-tetramethyl-1,3-benzenedipropionic acid)] 95%](/deepweb/assets/sigmaaldrich/product/structures/102/178/d1171a49-0358-406b-8b32-04324dbf9c02/640/d1171a49-0358-406b-8b32-04324dbf9c02.png)