214027
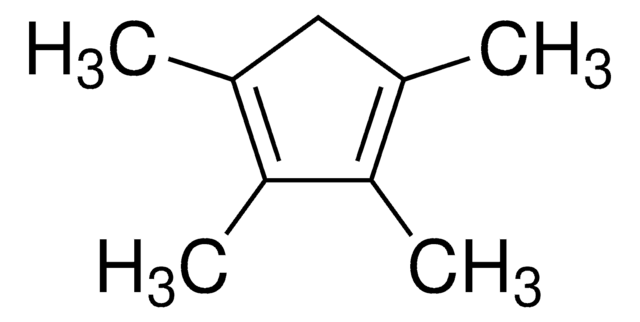
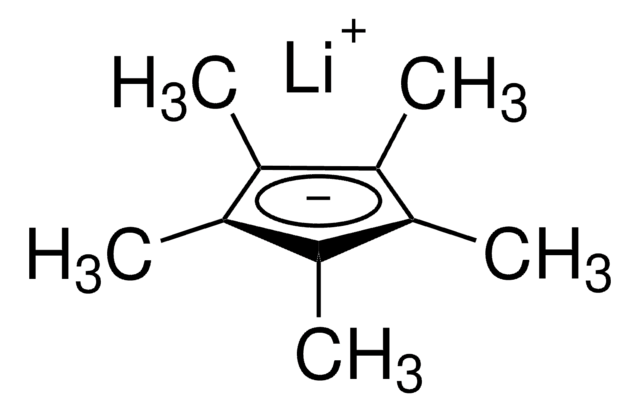
1,2,3,4,5-Pentamethylcyclopentadiene
95%
Synonym(s):
1,2,3,4,5-Pentamethyl-1,3-cyclopentadiene
About This Item
Recommended Products
Quality Level
assay
95%
refractive index
n20/D 1.474 (lit.)
bp
58 °C/13 mmHg (lit.)
density
0.87 g/mL at 25 °C (lit.)
SMILES string
CC1C(C)=C(C)C(C)=C1C
InChI
1S/C10H16/c1-6-7(2)9(4)10(5)8(6)3/h6H,1-5H3
InChI key
WQIQNKQYEUMPBM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Growth modifier chemical, during metal organic chemical vapour deposition of iron from iron pentacarbonyl.
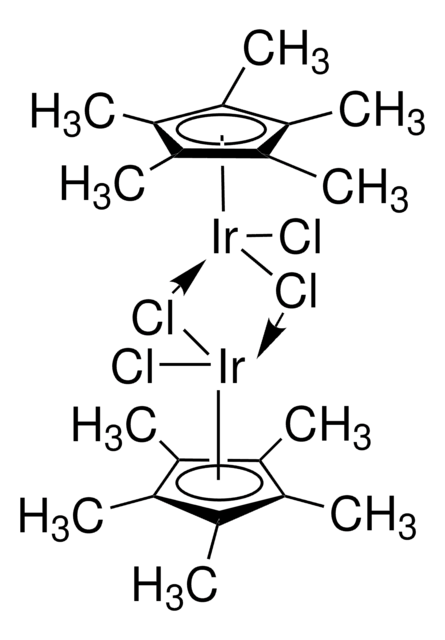
- Ligand in "one-pot" iridium-catalyzed transformation of alcohols to amides via the intermediacy of oximes.
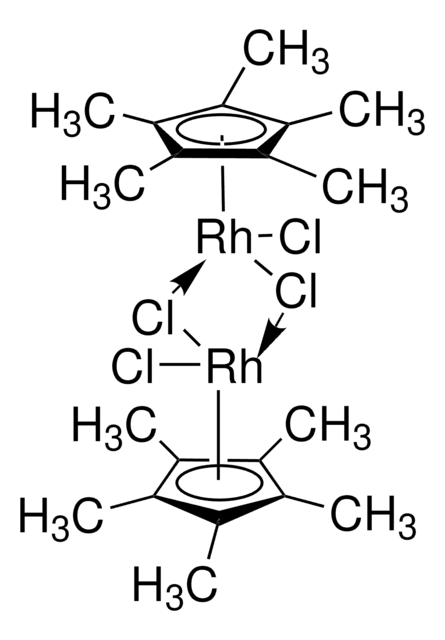
- Raw material for the synthesis of [Cp*Rh(bpy)H2O]2+ (Cp* = pentamethylcyclopentadienyl, bpy = 2,2′-bipyridyl), an electron mediator in the regeneration process of NADH.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service