357537
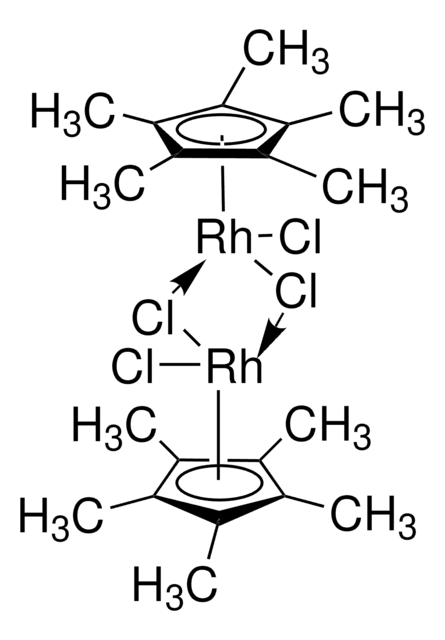
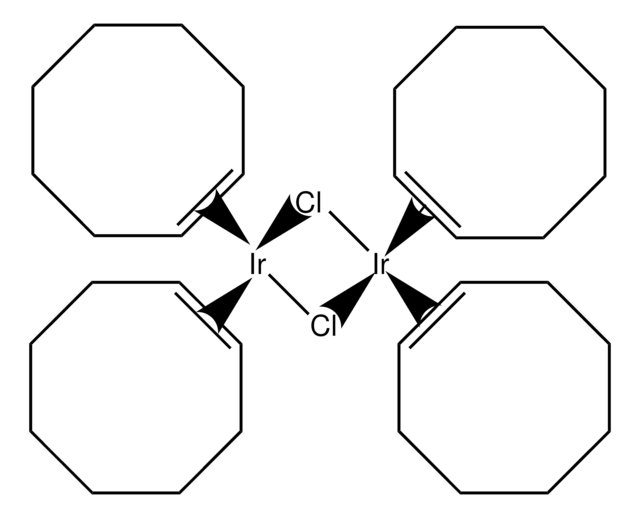
Pentamethylcyclopentadienyliridium(III) chloride,dimer
96%
Synonym(s):
di-μ-chlorodichlorobis[(1,2,3,4,5-η)-1,2,3,4,5-pentamethyl-2,4-cyclopentadien-1-yl]di-iridium
About This Item
Recommended Products
Quality Level
assay
96%
form
solid
reaction suitability
core: iridium
reagent type: catalyst
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
greener alternative category
SMILES string
Cl[Ir]Cl.Cl[Ir]Cl.C[C]1[C](C)[C](C)[C](C)[C]1C.C[C]2[C](C)[C](C)[C](C)[C]2C
InChI
1S/2C10H15.4ClH.2Ir/c2*1-6-7(2)9(4)10(5)8(6)3;;;;;;/h2*1-5H3;4*1H;;/q;;;;;;2*+2/p-4
InChI key
JDOHSXHCQARCAW-UHFFFAOYSA-J
General description
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalytic efficiency. Click here for more information.
Application
Metal-Brønsted Acid Cooperative Catalysis for Asymmetric Reductive Amination
Development of GSK's reagent guides – embedding sustainability into reagent selection
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service