226769
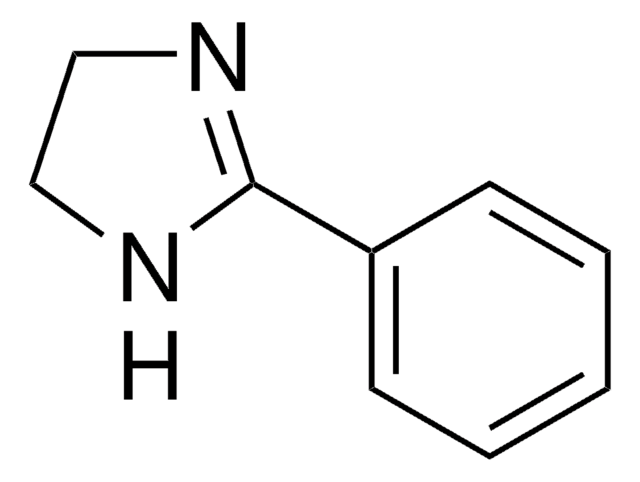
2-Phenylimidazole
98%
Synonym(s):
2-Phenyl-1H-imidazole
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C9H8N2
CAS Number:
Molecular Weight:
144.17
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
solid
mp
142-148 °C (lit.)
functional group
phenyl
SMILES string
c1ccc(cc1)-c2ncc[nH]2
InChI
1S/C9H8N2/c1-2-4-8(5-3-1)9-10-6-7-11-9/h1-7H,(H,10,11)
InChI key
ZCUJYXPAKHMBAZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Phenylimidazole was used to prepare complex compounds with ruthenium(III).
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
392.0 °F
flash_point_c
200 °C
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Lourdes Infantes et al.
The journal of physical chemistry. A, 110(7), 2535-2544 (2006-02-17)
The enthalpies of combustion, heat capacities, enthalpies of sublimation and enthalpies of formation of 2-tert-butylbenzimidazole (2tBuBIM) and 2-phenylimidazole (2PhIM) are reported and the results compared with those of benzene derivatives and a series of azoles (imidazoles, pyrazoles, benzimidazoles and indazoles).
T Shimizu et al.
Biochemistry, 30(6), 1490-1496 (1991-02-12)
Interactions of various axial ligands with cytochrome P-450d wild type, proximal mutants (Lys453Glu, Ile460Ser), and putative distal mutants (Glu318Asp, Thr319Ala, Thr322Ala) expressed in yeast were studied with optical absorption spectroscopy. P-450d wild type and all five mutants were purified essentially
A Nikolova et al.
Arzneimittel-Forschung, 51(9), 758-762 (2001-10-20)
Complex compounds of ruthenium(III) with 1,2-dimethylimidazole (CAS 1739-84-0), 2-phenylimidazole (CAS 670-96-2) and 2-aminobenzimidazole (CAS 934-32-7) were prepared and were characterised by physicochemical methods. Coordination sites were determined. The complexes were tested for cytotoxic activity using MTT (3-(4,5-dimethyithiazol-2-yl)-2,5-diphenyltetrazolium bromide) dye-reduction assay
Manoj Kumar Dalai et al.
Indian journal of biochemistry & biophysics, 43(2), 105-118 (2006-09-08)
Considering the potential of peripheral benzodiazepine receptor (PBR) ligands in therapeutic applications and clinical benefit in the management of a large spectrum of different indications, quantitative structure-activity relationship (QSAR) study has been attempted to explore the structural and physicochemical requirements
T Shimizu et al.
Biochimica et biophysica acta, 995(2), 116-121 (1989-04-06)
By site-directed mutagenesis, we made several cytochrome P-450d (P-450d) mutants as follows: Asn310Phe (D13), Ile312Leu (D14), Glu318Asp (D15), Val320Ile (D16), Phe325Thr (D19), Asn310Phe,Ile312Leu (M6), Glu318Asp,Val320Ile (M7), Phe325Thr, Glu318Asp (M3). This region (Asn-310-Phe-325) is supposed to be located in the distal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service