All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H8N2
CAS Number:
Molecular Weight:
144.17
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
form
liquid
bp
142 °C/15 mmHg (lit.)
mp
13 °C (lit.)
density
1.14 g/mL at 25 °C (lit.)
SMILES string
c1ccc(cc1)-n2ccnc2
InChI
1S/C9H8N2/c1-2-4-9(5-3-1)11-7-6-10-8-11/h1-8H
InChI key
SEULWJSKCVACTH-UHFFFAOYSA-N
General description
1-Phenylimidazole is an imidazole derivative. It induces 7-ethoxyresorufin-O-deethylase (EROD) activity in rainbow trout (Oncorhynchus mykiss) hepatocytes. The S(1)→S(0) transition of 1-phenylimidazole has been investigated in a supersonic jet expansion by resonant two-photon ionization. 1-Phenylimidazole is reported to be inhibitor of calmodulin-dependent nitric-oxide synthase from bovine brain and GHs pituitary cells.
Application
1-Phenylimidazole is a suitable reagent used to investigate its effect on the citrulline formation by bovine brain nitric-oxide synthase.
signalword
Warning
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Priyadarshini Balaraman et al.
Biochimica et biophysica acta. General subjects, 1863(2), 304-312 (2018-11-06)
The camphor-degrading microorganism, Pseudomonas putida strain ATCC 17453, is an aerobic, gram-negative soil bacterium that uses camphor as its sole carbon and energy source. The genes responsible for the catabolic degradation of camphor are encoded on the extra-chromosomal CAM plasmid.
A J Green et al.
Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry, 6(5-6), 523-533 (2001-07-27)
The bioI gene has been sub-cloned and over-expressed in Escherichia coli, and the protein purified to homogeneity. The protein is a cytochrome P450, as indicated by its visible spectrum (low-spin haem iron Soret band at 419 nm) and by the
A Jos et al.
Toxicology in vitro : an international journal published in association with BIBRA, 21(7), 1307-1310 (2007-05-25)
The classical pathway for induction of cytochrome P4501A (CYP1A) by xenobiotics is ligand binding to the aryl hydrocarbon receptor (AhR). However, several studies with mammalian cell systems point out a range of xenobiotics including imidazole derivatives, which are able to
Filippo Monti et al.
Inorganic chemistry, 54(6), 3031-3042 (2015-03-06)
A series of cationic iridium(III) complexes with two carbene-based cyclometalating ligands and five different N^N bipyridine and 1,10-phenanthroline ancillary ligands is presented. For the first time--in the frame of a rarely studied class of bis(heteroleptic) iridium complexes with two carbene-based
M R Anari et al.
Chemical research in toxicology, 9(6), 924-931 (1996-09-01)
Organic hydroperoxides are believed to be primarily detoxified in cells by the GSH peroxidase/GSSG reductase system and activated to cytotoxic radical species by non-heme iron. However, organic hydroperoxides seem to be bioactivated by cytochrome P450 (P450) in isolated hepatocytes as
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service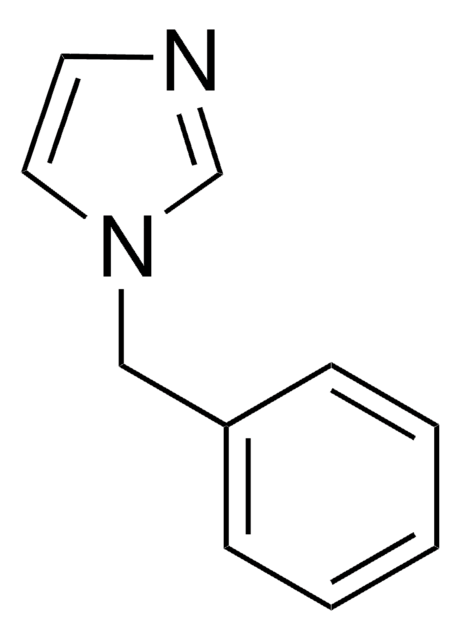








![1-[2-(Trifluoromethyl)phenyl]imidazole](/deepweb/assets/sigmaaldrich/product/structures/150/780/ea7e6b25-7659-422e-868c-8df7fd70d66e/640/ea7e6b25-7659-422e-868c-8df7fd70d66e.png)