244481
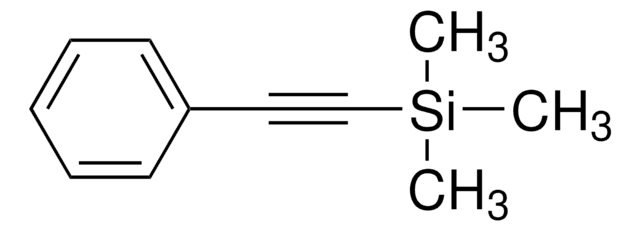
1-(Trimethylsilyl)propyne
99%
Synonym(s):
Trimethyl-1-propynylsilane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C≡CSi(CH3)3
CAS Number:
Molecular Weight:
112.24
Beilstein/REAXYS Number:
1071311
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
>1 (vs air)
Quality Level
assay
99%
form
liquid
refractive index
n20/D 1.417 (lit.)
bp
99-100 °C (lit.)
density
0.758 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CC#C[Si](C)(C)C
InChI
1S/C6H12Si/c1-5-6-7(2,3)4/h1-4H3
InChI key
DCGLONGLPGISNX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1-(Trimethylsilyl)propyne was used in the synthesis of highly substituted indenes via palladium-catalyzed carboannulation and indenones via a rhodium-catalyzed reaction with 2-bromophenylboronic acids.
signalword
Danger
hcodes
Hazard Classifications
Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
39.2 °F - closed cup
flash_point_c
4 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Journal of the American Chemical Society, 105, 7473-7473 (1983)
Tetrahedron Letters, 33, 5969-5969 (1992)
Yasuyuki Harada et al.
Journal of the American Chemical Society, 129(17), 5766-5771 (2007-04-10)
The Rh-catalyzed reaction of alkynes with 2-bromophenylboronic acids involves carbonylative cyclization to give indenones. The key steps in the reaction involve the addition of an arylrhodium(I) species to an alkyne and the oxidative addition of C-Br bonds on the adjacent
Daohua Zhang et al.
The Journal of organic chemistry, 72(1), 251-262 (2006-12-30)
The synthesis of highly substituted indenes has been achieved by three different transition metal-mediated methods. The first method involves the palladium-catalyzed carboannulation of internal alkynes. The second method utilizes a two-step approach, which involves first the palladium/copper-catalyzed cross-coupling of terminal
Journal of Polymer Science Part A: Polymer Chemistry, 25, 1353-1353 (1987)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service