247898
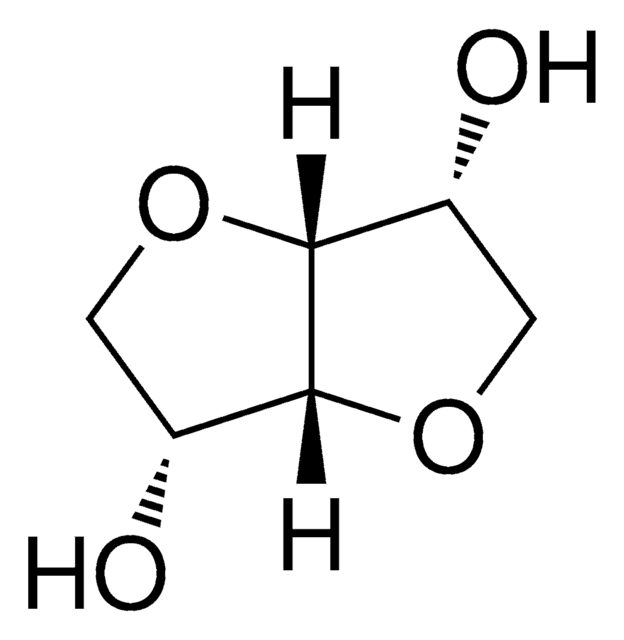
Isosorbide dimethyl ether
98%
Synonym(s):
DMI, Dimethyl isosorbide, 1,4:3,6-Dianhydro-2,5-di-O-methyl-D-glucitol, 1,4:3,6-Dianhydrosorbitol 2,5-dimethyl ether, 2,5-Di-O-methyl-1,4:3,6-dianhydro-D-glucitol
About This Item
Recommended Products
assay
98%
form
liquid
optical activity
[α]21/D +96.1°, c = 2 in chloroform
refractive index
n20/D 1.461 (lit.)
bp
93-95 °C/0.1 mmHg (lit.)
density
1.15 g/mL at 25 °C (lit.)
SMILES string
CO[C@H]1COC2[C@@H](COC12)OC
InChI
1S/C8H14O4/c1-9-5-3-11-8-6(10-2)4-12-7(5)8/h5-8H,3-4H2,1-2H3/t5-,6+,7-,8-/m1/s1
InChI key
MEJYDZQQVZJMPP-ULAWRXDQSA-N
Looking for similar products? Visit Product Comparison Guide
Application
General applications:
- DMI can be used as an alternative green solvent for epoxidation of cyclooctene[2], Baylis–Hillman reaction of isomaltulose-derived aldehydes with α,β-unsaturated ketones[3] and also in solid-phase synthesis.[4]
- It can be used as a coalescent for water-borne paints, where it acts as a co-solvent during the water loss phase and as a coalescing agent during the film formation.[5]
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
240.8 °F - closed cup
flash_point_c
116 °C - closed cup
ppe
Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service