263567
1,4-Bis(trimethylsilyl)butadiyne
98%, stable crystalline form of butadiyne
Synonym(s):
1,4-Bis(trimethylsilyl)-1,3-butadiyne, BTMSBD
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)3SiC≡CC≡CSi(CH3)3
CAS Number:
Molecular Weight:
194.42
Beilstein/REAXYS Number:
1758268
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
form
solid
refractive index
n20/D 1.384 (lit.)
mp
111-113 °C (lit.)
density
0.974 g/mL at 20 °C (lit.)
Storage temp.
2-8°C
SMILES string
C[Si](C)(C)C#CC#C[Si](C)(C)C
InChI
1S/C10H18Si2/c1-11(2,3)9-7-8-10-12(4,5)6/h1-6H3
Inchi Key
LBNVCJHJRYJVPK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1,4-Bis(trimethylsilyl)butadiyne can be used as a reagent to prepare:
- 1,1,3,4-Tetrasilyl-substituted 1,3-butadienes or 1,1,3,4-tetrasilyl-substituted 1,2-butadienes by hydrosilylation reaction using various hydridosilanes and catalysts.
- Glycosylated oligo(ethynylene)s using the Negishi reaction.
- (±) Falcarinol, a polyacetylene class of fatty alcohol.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A convenient Negishi protocol for the synthesis of glycosylated oligo (ethynylene) s
Hoheisel TN and Frauenrath H
Organic Letters, 10, 4525-4528 (2008)
A short synthesis of (+) and (-)-falcarinol
McLaughlin NP, et al.
Tetrahedron, 66, 9681-9687 (2010)
Hydrosilylation of 1, 4-Bis (trimethylsilyl) butadiyne and Silyl-Substituted Butenynes.
Kusumoto T, et al.
Bulletin of the Chemical Society of Japan, 65(5), 1280-1290 (1992)
Manuel M Bentlohner et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 23(67), 17089-17094 (2017-09-15)
We report on the synthesis and structure, as well as on the mechanism of formation of the [Ge
Hydrosilylation of 1, 4-bis (trimethylsilyl) butadiyne and silyl-substituted butenynes
Kusumoto T, et al.
Bulletin of the Chemical Society of Japan, 65, 1280-1290 (1992)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





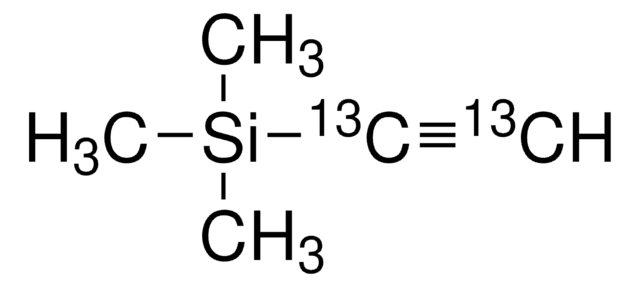

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)