275581
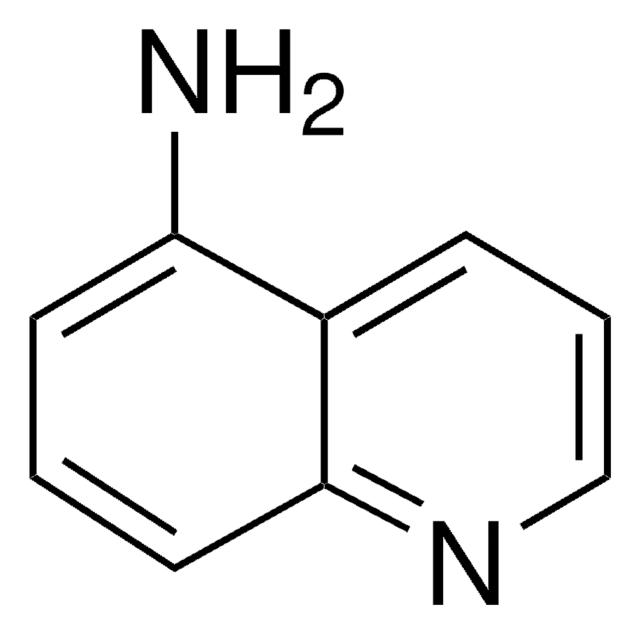
6-Aminoquinoline
98%
Synonym(s):
6-Quinolinamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H8N2
CAS Number:
Molecular Weight:
144.17
Beilstein/REAXYS Number:
113320
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
solid
bp
146 °C/0.3 mmHg (lit.)
mp
115-119 °C (lit.)
SMILES string
Nc1ccc2ncccc2c1
InChI
1S/C9H8N2/c10-8-3-4-9-7(6-8)2-1-5-11-9/h1-6H,10H2
InChI key
RJSRSRITMWVIQT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
6-Aminoquinoline was used as an internal standard in determining serum nicotine and cotinine simultaneously by using high-performance liquid chromatography (HPLC)-fluorometric detection with a postcolumn ultraviolet-photoirradiation system. It was also used as a fluorescent derivatizing agent for the detection of biochemicals and in the synthesis of tertiary N-methylated enaminonesa.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jong Bae Seo et al.
PloS one, 10(12), e0144432-e0144432 (2015-12-15)
Hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) of the plasma membrane by phospholipase C (PLC) generates two critical second messengers, inositol-1,4,5-trisphosphate and diacylglycerol. For the enzymatic reaction, PIP2 binds to positively charged amino acids in the pleckstrin homology domain of PLC. Here
Pierre-Jean Aragon et al.
Chemical & pharmaceutical bulletin, 52(6), 659-663 (2004-06-10)
Indoloquinoline alkaloid cryptolepine and pyridocarbazole alkaloid ellipticine are of great interest because in vitro and in vivo studies revealed their good cytotoxic properties. In order to obtain some biologically active analogs of these compounds, we developped a synthesis based on
Bryan P Ruddy et al.
Journal of controlled release : official journal of the Controlled Release Society, 306, 83-88 (2019-06-01)
Subcutaneous delivery of nicotine was performed using a novel electrically-operated needle-free jet injector, and compared to hypodermic needle delivery in a porcine model. Nicotine was delivered as a single, one-milligram dose into the abdominal skin, formulated as a 50 microliter
W Nashabeh et al.
Journal of chromatography, 600(2), 279-287 (1992-05-29)
The electrophoretic behavior of derivatized linear and branched oligosaccharides from various sources was examined in capillary zone electrophoresis with polyether-coated fused-silica capillaries. Two UV-absorbing (also fluorescent) derivatizing agents (2-aminopyridine and 6-aminoquinoline) were utilized for the electrophoresis and sensitive detection of
S A Cohen et al.
Analytical biochemistry, 211(2), 279-287 (1993-06-01)
A highly reactive amine derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, has been synthesized. In a rapid, one-step procedure, the compound reacts with amino acids to form stable unsymmetric urea derivatives which are readily amenable to analysis by reversed phase HPLC. Studies on
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service