324124
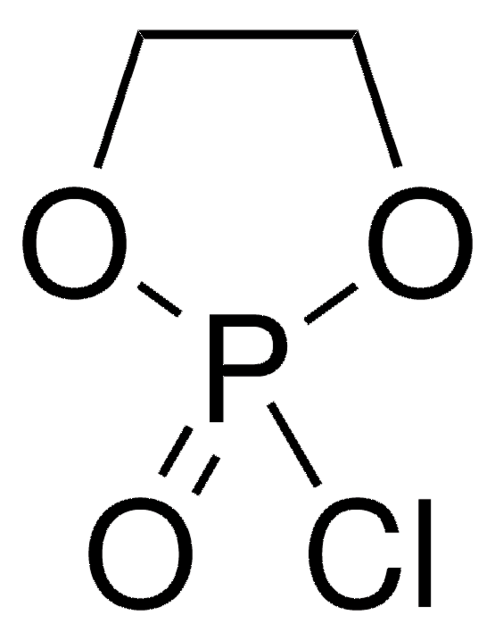
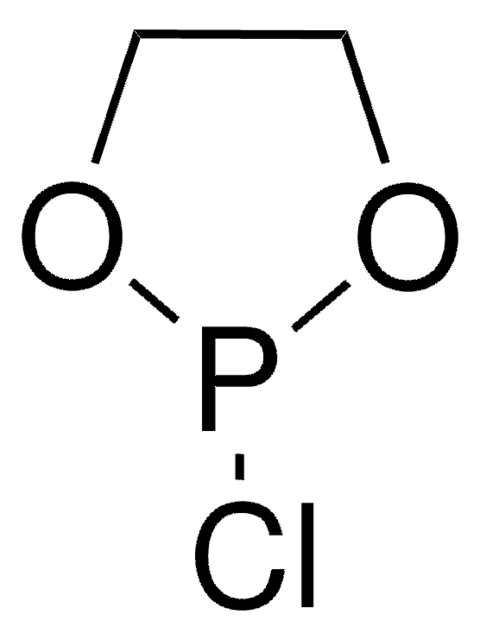
2-Chloro-1,3,2-benzodioxaphosphorin-4-one
95%
Synonym(s):
SalPCl, Salicyl chlorophosphite
About This Item
Recommended Products
Quality Level
assay
95%
form
solid
reaction suitability
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
bp
127-128 °C/11 mmHg (lit.)
mp
36-40 °C (lit.)
functional group
phosphine
storage temp.
2-8°C
SMILES string
ClP1OC(=O)c2ccccc2O1
InChI
1S/C7H4ClO3P/c8-12-10-6-4-2-1-3-5(6)7(9)11-12/h1-4H
InChI key
BVOITXUNGDUXRW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- In the phosphorylation and phosphitylation of alcohols.
- In the formation of H-phosphonates which are commonly utilized in the synthesis of nucleotides.
- To synthesize nucleoside triphosphates by treating with acyl protected nucleoside.
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
supp_hazards
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)