All Photos(1)
About This Item
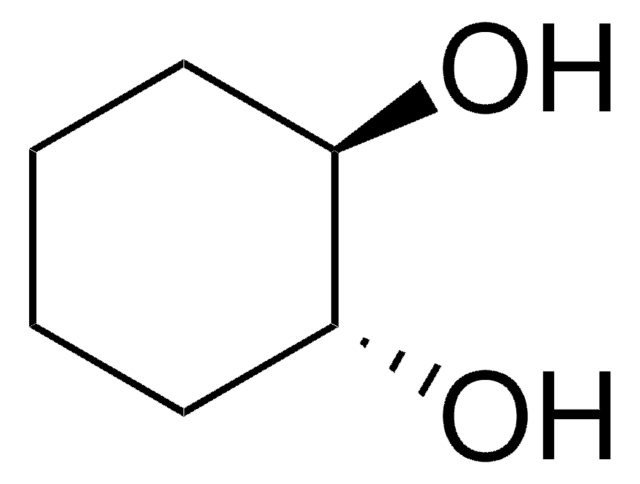
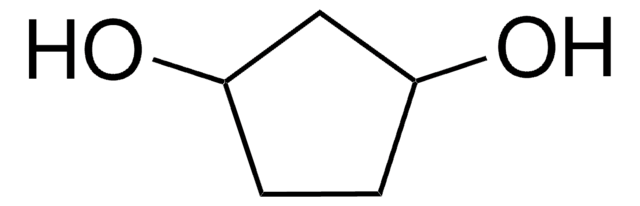
Linear Formula:
C6H10(OH)2
CAS Number:
Molecular Weight:
116.16
Beilstein/REAXYS Number:
1340578
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
mp
97-101 °C (lit.)
functional group
hydroxyl
SMILES string
O[C@@H]1CCCC[C@@H]1O
InChI
1S/C6H12O2/c7-5-3-1-2-4-6(5)8/h5-8H,1-4H2/t5-,6+
InChI key
PFURGBBHAOXLIO-OLQVQODUSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Core-shell-like silica nickel species nanoparticle catalyzed dehydrogenation of 1,2-cyclohexanediol to catechol is reported. Crystal structure of a Cr(V) complex with cis-1,2-cyclohexanediol is reported. Enzymatic oxidation of cis-1,2-cyclohexanediol by Gluconobacter oxydans (ATCC 621) is reported.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Oxidation of trans-and cis-1, 2-cyclohexanediol by Gluconobacter oxydans.
Adlercreutz P.
Applied Microbiology and Biotechnology, 30(3), 257-263 (1989)
Ruben Bartholomäus et al.
Inorganic chemistry, 52(8), 4282-4292 (2013-03-28)
The stabilization of Cr(V) by biological 1,2-diolato ligands, including carbohydrates, glycoproteins, and sialic acid derivatives, is likely to play a crucial role in the genotoxicity of Cr(VI) and has also been implicated in the antidiabetic effect of Cr(III). Previously, such
Bao-Hui Chen et al.
Dalton transactions (Cambridge, England : 2003), 44(3), 1023-1038 (2014-11-20)
A simple and convenient approach denoted as gel-deposition-precipitation (G-D-P) for the preparation of core-shell-like silica@nickel species nanoparticles was studied systematically. Core-shell-like silica@nickel species nanoparticles consisted of a Si-rich core and a Ni-rich shell. The G-D-P process included two steps: one
C B Shinisha et al.
Organic letters, 11(15), 3242-3245 (2009-09-02)
The relative energies of cyclohexane-1,2-diols and chiral tetrapeptide (2 (Boc) or 3 (Moc)) complexes calculated using DFT indicate a thermodynamic preference for chiral recognition toward (1R,2R)(e,e)-alpha isomer. The barrier for stereoselective acyl transfer is identified as lower for trans-(1R,2R)-cyclohexane-1,2-diol, leading
R J Swift et al.
Applied microbiology and biotechnology, 55(6), 721-726 (2001-08-30)
Benzene dioxygenase (BDO; EC 1.14.12.3) from Pseudomonas putida ML2 dihydroxylates benzene to produce cis-1,2-dihydroxy-cyclohexa-3,5-diene. As well as oxidising benzene and toluene, cell-free extracts of Escherichia coli JM109 expressing recombinant BDO oxidised cyclohexene, 1-methylcyclohexene and 3-methylcyclohexene. In an attempt to construct
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service