C102504
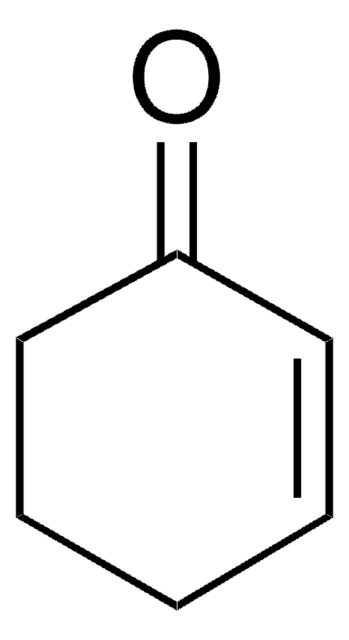
Cyclohexene oxide
98%
Synonym(s):
1,2-Epoxycyclohexane, 7-Oxabicyclo[4.1.0]heptane
About This Item
Recommended Products
Quality Level
assay
98%
form
liquid
autoignition temp.
703 °F
expl. lim.
12.36 %
refractive index
n20/D 1.452 (lit.)
bp
129-130 °C (lit.)
density
0.97 g/mL at 25 °C (lit.)
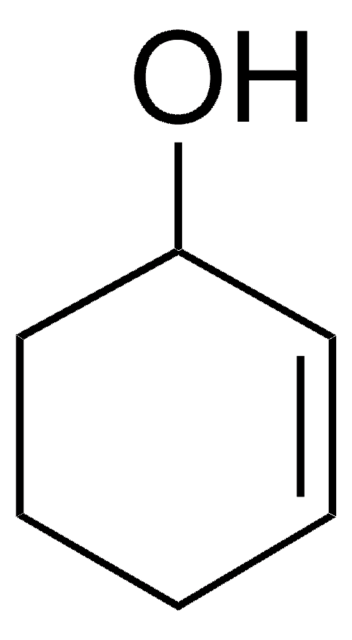
SMILES string
C1CCC2OC2C1
InChI
1S/C6H10O/c1-2-4-6-5(3-1)7-6/h5-6H,1-4H2
InChI key
ZWAJLVLEBYIOTI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Polymeric carbon nitride with internal np homojunctions for efficient photocatalytic CO2 reduction coupled with cyclohexene oxidation: This study focuses on the use of cyclohexene oxide in the context of photocatalytic CO2 reduction, highlighting the application of polymeric carbon nitride as a catalyst. The process shows how cyclohexene oxide can be efficiently converted in a coupled reaction that also addresses environmental concerns through CO2 reduction (W Zhen et al., 2021).
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Muta. 2 - Skin Corr. 1B
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![7-Oxabicyclo[4.1.0]heptan-2-one 98%](/deepweb/assets/sigmaaldrich/product/structures/209/639/448778d7-ca19-409d-a52e-8d2866c49812/640/448778d7-ca19-409d-a52e-8d2866c49812.png)







![7-Oxabicyclo[2.2.1]heptane 98%](/deepweb/assets/sigmaaldrich/product/structures/377/935/931d29d9-08c9-492a-b42e-3f8f5a20f595/640/931d29d9-08c9-492a-b42e-3f8f5a20f595.png)