374318
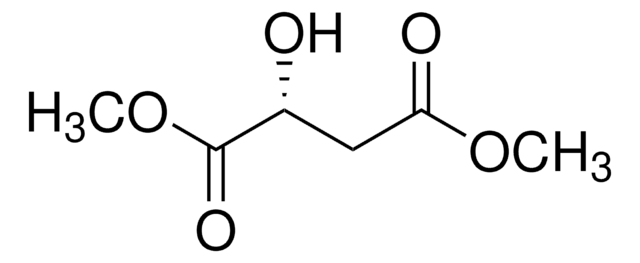
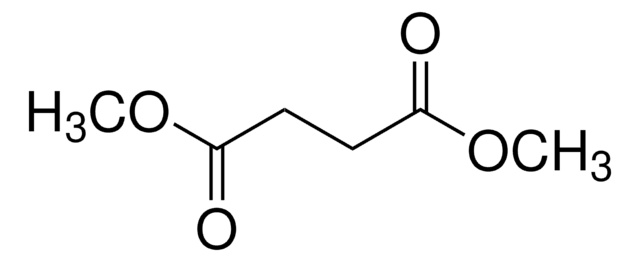
Dimethyl (S)-(−)-malate
98%
Synonym(s):
Dimethyl (S)-2-hydroxysuccinate, Dimethyl L-malate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3O2CCH2CH(OH)CO2CH3
CAS Number:
Molecular Weight:
162.14
Beilstein/REAXYS Number:
1724363
EC Number:
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
optical activity
[α]20/D −6.5°, neat
refractive index
n20/D 1.435 (lit.)
density
1.223 g/mL at 25 °C (lit.)
functional group
ester
hydroxyl
storage temp.
2-8°C
SMILES string
COC(=O)C[C@H](O)C(=O)OC
InChI
1S/C6H10O5/c1-10-5(8)3-4(7)6(9)11-2/h4,7H,3H2,1-2H3/t4-/m0/s1
InChI key
YSEKNCXYRGKTBJ-BYPYZUCNSA-N
Application
Dimethyl (S)-(-)-malate may be used as a starting material to synthesize (-)-tulipalin B. The selective reduction of its ester group to alcohol can be accomplished using borane-dimethyl sulfide complex (BMS) in the presence of sodium tetrahydroborate.
This chiral synthon has been used to prepare cytochrome P450 metabolites of arachidonic acid, and cyclic sulfolanes with HIV-1 protease inhibition potential.
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 3 - Skin Sens. 1
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
131.0 °F
flash_point_c
55 °C
ppe
Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cyclic sulfolanes as novel and high affinity P2 ligands for HIV-1 protease inhibitors.
A K Ghosh et al.
Journal of medicinal chemistry, 36(7), 924-927 (1993-04-02)
Falck, J.R. et al.
Tetrahedron Letters, 33, 4893-4893 (1992)
Use of enzymic hydrolysis of dimethyl malates for a short synthesis of tulipalin B and of its enantiomer.
Papageorgiou C and Benezra C.
The Journal of Organic Chemistry, 50(7), 1144-1145 (1985)
Rajesh Gupta et al.
The Journal of biological chemistry, 293(47), 18086-18098 (2018-09-20)
Secreted proteins are important metabolic regulators in both healthy and disease states. Here, we sought to investigate the mechanism by which the secreted protein complement 1q-like-3 (C1ql3) regulates insulin secretion from pancreatic β-cells, a key process affecting whole-body glucose metabolism.
Combination of borane-dimethyl sulfide complex with catalytic sodium tetrahydroborate as a selective reducing agent for a-hydroxy esters. Versatile chiral building block from (s)-(-)-malic acid.
Saito S, et al.
Chemistry Letters (Jpn), 13(8), 1389-1392 (1984)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service