378658
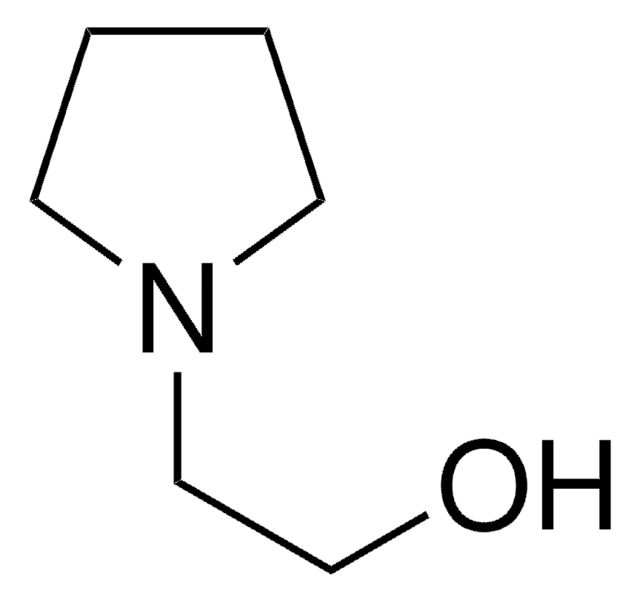
1-(2-Hydroxyethyl)-2-imidazolidinone solution
75% in H2O
Synonym(s):
1-(Hydroxyethyl)ethyleneurea, NSC 5775
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H10N2O2
CAS Number:
Molecular Weight:
130.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
liquid
Quality Level
concentration
75% in H2O
refractive index
n20/D 1.466
density
1.19 g/mL at 25 °C
functional group
hydroxyl
SMILES string
OCCN1CCNC1=O
InChI
1S/C5H10N2O2/c8-4-3-7-2-1-6-5(7)9/h8H,1-4H2,(H,6,9)
InChI key
HBAIZGPCSAAFSU-UHFFFAOYSA-N
General description
1-(2-Hydroxyethyl)-2-imidazolidinone has been identified as monoethanolamine (MEA) degradation product in IMC Chemicals Facility in Trona, CA plant performing CO2 capture from flue gas. It was also identified from the MEA reclaimer from a CO2 capture facility product. It was also identified as the most stable thermal degradation product of MEA.
Application
Reagent for synthesis of HIV-1 integrase inhibitors
Reactant for synthesis of:
MDM2-p53 protein-protein interaction inhibitors
Amino acid-derived heterocycles
dopamine D-2 and serotonin 5-HT2 antagonists
Reactant for synthesis of:
MDM2-p53 protein-protein interaction inhibitors
Amino acid-derived heterocycles
dopamine D-2 and serotonin 5-HT2 antagonists
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Degradation pathways for monoethanolamine in a CO2 capture facility.
Strazisar BR, et al.
Energy and Fuels, 17(4), 1034-1039 (2003)
Degradation of monoethanolamine used in carbon dioxide capture from flue gas of a coal-fired electric power generating station.
Strazisar BR, et al.
Journal of Energy & Environmental Research, 1(1), 32-39 (2001)
Thermal degradation of monoethanolamine and its effect on CO2 capture capacity.
Zoannou K-S, et al.
International Journal of Greenhouse Gas Control, 17, 423-440 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service