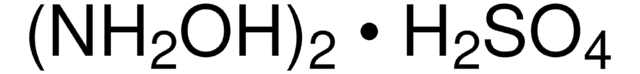379921
Hydroxylamine hydrochloride
99.995% trace metals basis
Synonym(s):
Hydroxylammonium chloride
About This Item
Recommended Products
assay
99.995% trace metals basis
form
powder, crystals or chunks
pH
2.5-3.5 (20 °C, 50 g/L)
mp
155-157 °C (dec.) (lit.)
density
1.67 g/mL at 25 °C (lit.)
SMILES string
Cl.NO
InChI
1S/ClH.H3NO/c;1-2/h1H;2H,1H2
InChI key
WTDHULULXKLSOZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Organosilane amines as potent inhibitors and structural probes of influenza A virus M2 proton channel[1]
- Lamellarin D analogues as inibitors of topoisomerase I and potential antitumor agents[2]
- Azapeptide tocolytic agents as inhibitors of prostaglandin F2α receptor for preventing preterm labor[3]
- Thiazolidinones spiro-fused to indolin-2-ones as potent and selective inhibitors of Mycobacterium tuberculosis protein tyrosine phosphatase B[4]
- Orally bioavailable quinoline-based antidiabetic dipeptidyl peptidase IV inhibitors targeting Lys554[5]
- Pyrimidine nucleoside derivatives with nitric oxide donors as antiviral agents[6]
- Benzyladenosine compounds targeting adenosine A2A receptor and adenosine transporter for neuroprotection[7]
- Naphthol derivatives as inhibitors of the vanilloid receptor TRPV1 with improved potency in rat cystometry models of urinary incontinence[8]
Biochem/physiol Actions
signalword
Warning
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Carc. 2 - Eye Irrit. 2 - Met. Corr. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 2 Oral
target_organs
spleen
Storage Class
4.1A - Other explosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service