All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H10O
CAS Number:
Molecular Weight:
146.19
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
form
liquid
refractive index
n20/D 1.555 (lit.)
bp
93-95 °C/4 mmHg (lit.)
density
1.064 g/mL at 25 °C (lit.)
functional group
ketone
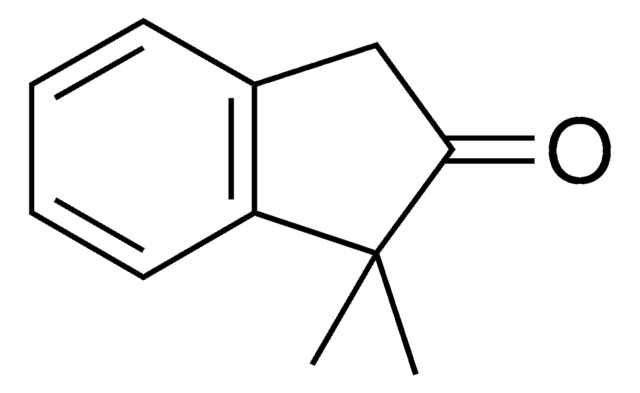
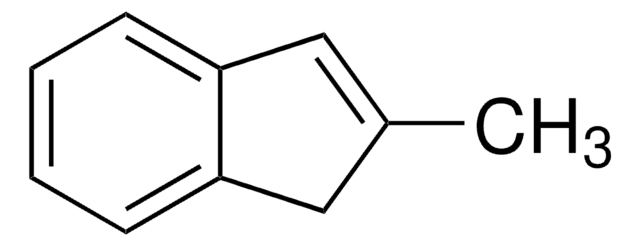
SMILES string
CC1Cc2ccccc2C1=O
InChI
1S/C10H10O/c1-7-6-8-4-2-3-5-9(8)10(7)11/h2-5,7H,6H2,1H3
InChI key
BEKNOGMQVKBMQN-UHFFFAOYSA-N
General description
2-Methyl-1-indanone, a α-benzocycloalkenone, is a derivative of 1-indanone. Its synthesis has been reported. The enzymatic dynamic kinetic resolution (DKR) of racemic 2-methyl-1-indanone has been studied. The asymmetric α-arylation and hydroxymethylation of 2-methyl-1-indanone has been reported. It participated in the synthesis of 2-methyl-6-carboxyazulene.
Application
2-Methyl-1-indanone may be used as a starting material in the synthesis of β-benzocycloalkenone. It may be used in the synthesis of the following:
- cyclohex-2-en-1-yl 2-methyl-1H-inden-3-yl carbonate
- 2-hydroxy-2-methyl-1-indanone
- O-alkoxycarbonylation of lithium enolates
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Michael W Justik et al.
Molecules (Basel, Switzerland), 10(1), 217-225 (2007-11-17)
The conversion of alpha-benzocycloalkenones to homologous beta-benzocyclo-alkenones containing six, seven and eight-membered rings is reported. This was accomplished via a Wittig olefination-oxidative rearrangement sequence using[hydroxy(tosyloxy)iodo]-benzene (HTIB) is the oxidant, that enables the synthesis of regioisomeric pairs of methyl-substituted beta-benzocycloalkenones. The
Cyclophanes. 9. anti-[2.2](2, 6) Azulenophane. Synthesis and charge-transfer interaction.
Luhowy R and Keehn PM.
Journal of the American Chemical Society, 99(11), 3797-3805 (1977)
Taku Kitanosono et al.
Chemistry, an Asian journal, 10(1), 133-138 (2014-10-29)
Enzymes exhibit overwhelmingly superior catalysis compared with artificial catalysts. Current strategies to rival enzymatic catalysis require unmodified or minimally modified structures of active sites, gigantic molecular weight, and sometimes the use of harsh conditions such as extremely low temperatures in
Selective and easy preparation of enol carbonates of α-disubstituted aryl ketones from their lithium enolates.
Aboulhoda SJ, et al.
Tetrahedron Letters, 36(27), 4795-4796 (1995)
Amino alcohol-mediated enantioselective syntheses of α-substituted indanones and tetralones, ammonium enolates as key intermediates.
Muzart J.
Tetrahedron Asymmetry, 25(9), 697-704 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service