I2304
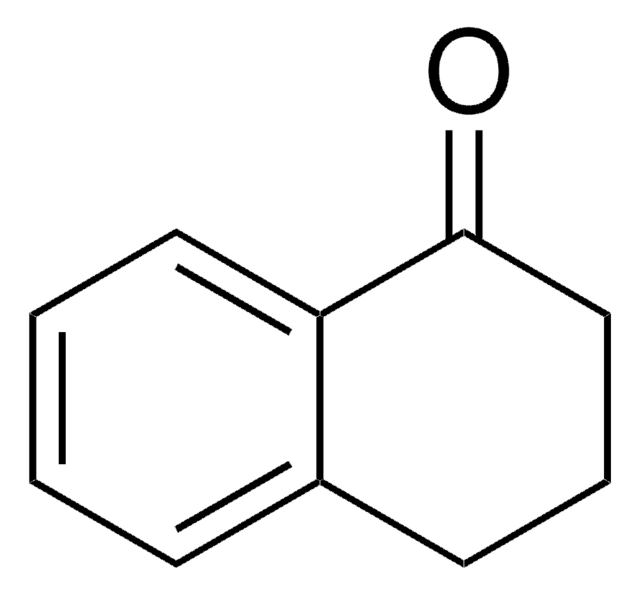
1-Indanone
ReagentPlus®, ≥99%
Synonym(s):
1-Oxoindane, α-Hydrindone
About This Item
Recommended Products
product line
ReagentPlus®
assay
≥99%
bp
243-245 °C (lit.)
mp
38-40 °C (lit.)
density
1.103 g/mL at 25 °C (lit.)
SMILES string
O=C1CCc2ccccc12
InChI
1S/C9H8O/c10-9-6-5-7-3-1-2-4-8(7)9/h1-4H,5-6H2
Inchi Key
QNXSIUBBGPHDDE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- 11H-Indeno-[1,2-b]-quinolin-10-ylamine, a potential acetylcholinesterase inhibitor for the treatment of Alzheimer′s disease.[1]
- CoumBARAC (coumarin fused biarylazacyclooctynone) for fluorogenic real-time imaging of azide-labeled biomolecules.[2]
- Light emitting, organic semiconductors such as 4-pyrenyl-2-piperidin-1-yl-9H-fluorene-1-carbonitrile[3] and bisindenoanthrazolines[4][5] for organic light emitting diode (OLED) applications.
It can also be used to build other indan-based systems like truxene[6], prekinamycin[7], indan-C60[8] and biindanylidenes.[9]
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
233.0 °F - closed cup
flash_point_c
111.67 °C - closed cup
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service