40407
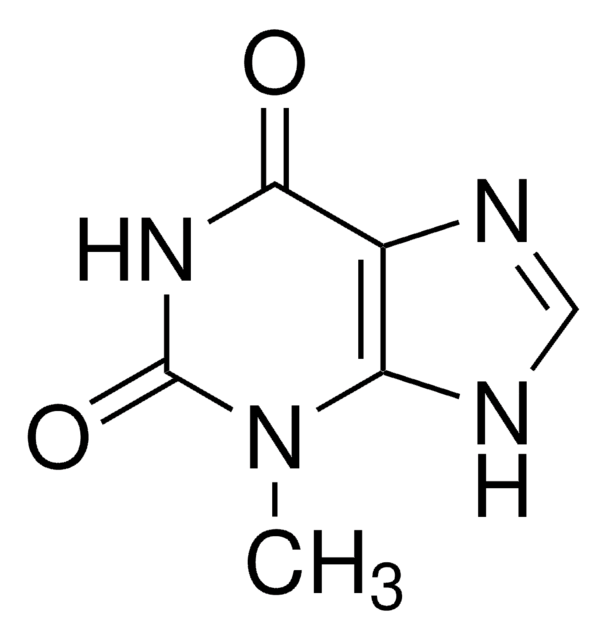
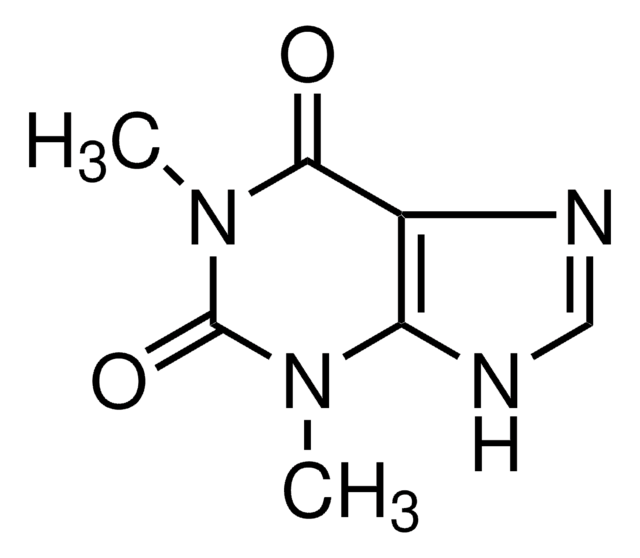
1,7-Dimethyluric acid
≥97.0% (HPLC)
Synonym(s):
1,7-Dimethyl-2,6,8-trihydroxypurine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H8N4O3
CAS Number:
Molecular Weight:
196.16
Beilstein/REAXYS Number:
219682
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥97.0% (HPLC)
SMILES string
CN1C(=O)NC2=C(N(C)C(=O)N2)C1=O
InChI
1S/C7H8N4O3/c1-10-3-4(8-6(10)13)9-7(14)11(2)5(3)12/h1-2H3,(H,8,13)(H,9,14)
InChI key
NOFNCLGCUJJPKU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,7-Dimethyluric acid is an important metabolite of caffeine. Electrochemical oxidation of 1,7-dimethyluric acid was studied over a wide pH range of 2.2-10.3 at solid electrodes.
Application
1,7-Dimethyluric acid is the suitable reagent used for the simultaneous determination of plasma levels of theophylline and its metabolites without interference from caffeine or caffeine metabolites by HPLC.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J Kizu et al.
Biomedical chromatography : BMC, 13(1), 15-23 (1999-04-07)
A high performance liquid chromatography (HPLC) method has been developed for the simultaneous determination of plasma levels of theophylline and its metabolites without interference from caffeine or caffeine metabolites. The method is simple and of practical use because it is
Electrochemical and peroxidase catalysed oxidation of 1, 7-dimethyluric acid and effect of methyl groups on the oxidation mechanism.
Goyal RN, et al.
J. Chem. Soc. Perkin Trans. II, 6, 1153-1159 (1996)
E Asprodini et al.
The Journal of pharmacology and experimental therapeutics, 368(2), 262-271 (2018-12-29)
The purpose of the study was to determine whether the in vivo activities of drug-metabolizing enzymes CYP1A2 and CYP2A6, xanthine oxidase (XO), and N-acetyltransferase-2 (NAT2) vary across the menstrual cycle. Forty-two healthy women were studied at early follicular phase (EFP:
M Vincent-Viry et al.
Genetic epidemiology, 11(2), 115-129 (1994-01-01)
Human acetylation phenotypes were determined with caffeine (137X) as the test substance, improved by measuring urinary caffeine metabolites with a previously described HPLC method. Caffeine, 5-acetylamino-6-formylamino-3-methyluracil (AFMU), 1-methylxanthine (IX), 1-methyluric acid (IU), 1,7-dimethylxanthine (17X), and 1,7-dimethyluric acid (17U) were quantified.
M E Campbell et al.
Clinical pharmacology and therapeutics, 42(2), 157-165 (1987-08-01)
Systemic caffeine clearance and urinary metabolite profiles were determined in 15 subjects with diverse exposure histories to cytochrome P-450 inducers (cigarette smoke) and inhibitors (oral contraceptive steroids). A correlation was observed between caffeine clearance and a urinary ratio based on
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service