All Photos(1)
About This Item
Empirical Formula (Hill Notation):
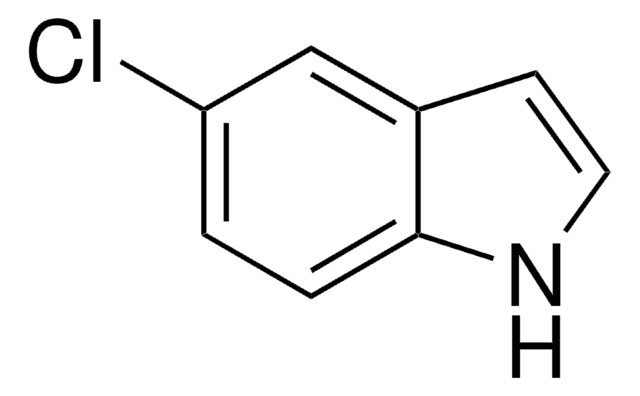
C8H6ClN
CAS Number:
Molecular Weight:
151.59
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
bp
90-95 °C/0.25 mmHg (lit.)
mp
55-58 °C (lit.)
functional group
chloro
SMILES string
Clc1cccc2cc[nH]c12
InChI
1S/C8H6ClN/c9-7-3-1-2-6-4-5-10-8(6)7/h1-5,10H
InChI key
WMYQAKANKREQLM-UHFFFAOYSA-N
General description
7-Chloroindole is an indole derivative. It has been synthesized from 2,3-dihydroindole.
Application
7-Chloroindole may be used in the preparation of 1-methyl-7-chloroindole and glycosylated 7-chloroindole-3-acetamide.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of rebeccamycin and 11-dechlororebeccamycin.
Faul MM, et al.
The Journal of Organic Chemistry, 64(7), 2465-2470 (1999)
Xin Teng et al.
Bioorganic & medicinal chemistry letters, 15(22), 5039-5044 (2005-09-13)
Necroptosis is a regulated caspase-independent cell death mechanism that results in morphological features resembling necrosis. It can be induced in a FADD-deficient variant of human Jurkat T cells treated with TNF-alpha. 5-(1H-Indol-3-ylmethyl)-2-thiohydantoins and 5-(1H-indol-3-ylmethyl)hydantoins were found to be potent necroptosis
The chemistry of indoles. XXXIX. A facile synthetic method for 7-substituted indoles.
Somei M, et al.
Chemical & Pharmaceutical Bulletin, 35(8), 3146-3154 (1987)
Jeongchan Lee et al.
Nature chemical biology, 17(1), 104-112 (2020-11-04)
Tyrian purple, mainly composed of 6,6'-dibromoindigo (6BrIG), is an ancient dye extracted from sea snails and was recently demonstrated as a biocompatible semiconductor material. However, its synthesis remains limited due to uncharacterized biosynthetic pathways and the difficulty of regiospecific bromination.
Chaitany Jayprakash Raorane et al.
Biomolecules, 10(8) (2020-08-23)
Multi-drug resistant Acinetobacter baumannii is well-known for its rapid acclimatization in hospital environments. The ability of the bacterium to endure desiccation and starvation on dry surfaces for up to a month results in outbreaks of health care-associated infections. Previously, indole
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service