489409
Fluoromethane-13C
99 atom % 13C
Synonym(s):
Methyl-13C fluoride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
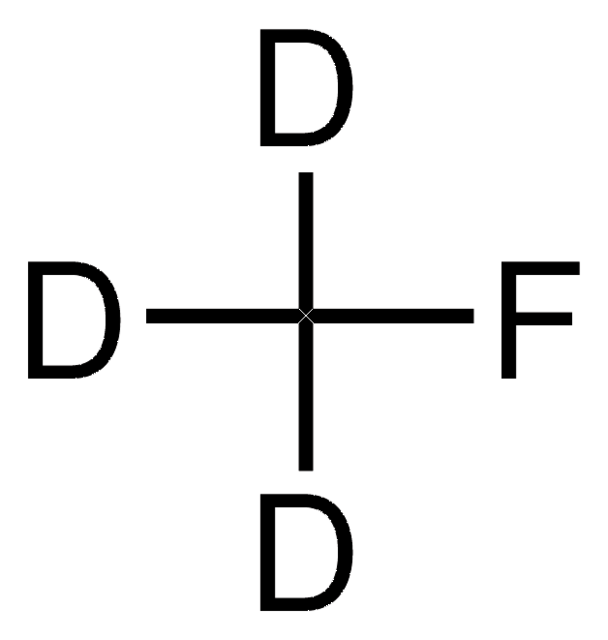
Linear Formula:
13CH3F
CAS Number:
Molecular Weight:
35.03
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.12
Recommended Products
vapor density
1.231 (vs air)
Quality Level
vapor pressure
44.2 atm ( 21.1 °C)
isotopic purity
99 atom % 13C
bp
−77.9 °C (lit.)
mp
−141.8 °C (lit.)
mass shift
M+1
SMILES string
[13CH3]F
InChI
1S/CH3F/c1-2/h1H3/i1+1
InChI key
NBVXSUQYWXRMNV-OUBTZVSYSA-N
Related Categories
Packaging
100 mL quantity is packaged in a 25 mL Sure/Pac™ cylinder with brass 1/4" male NPT valve. Nominal gas pressure at 21ºC is 32 psig for 100 mL. This pressure is slightly above atmospheric pressure. Care must be taken when extracting this product from the cylinder.
This product may be available from bulk stock and can be packaged on demand. For information on pricing, availability and packaging, please contact Stable Isotopes Customer Service.
Recommended products
Brass hose adapter Z146811 or brass body mini gas regulator Z513539 is recommended.
Legal Information
Sure/Pac is a trademark of Sigma-Aldrich Co. LLC
hose barb
Product No.
Description
Pricing
regulator
Product No.
Description
Pricing
signalword
Danger
hcodes
Hazard Classifications
Flam. Gas 1 - Press. Gas Liquefied gas
Storage Class
2A - Gases
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, multi-purpose combination respirator cartridge (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Waldemar Hujo et al.
Physical chemistry chemical physics : PCCP, 13(31), 13942-13950 (2011-05-20)
Potential energy curves for five complexes with weak to medium strong hydrogen bonds have been computed with dispersion corrected DFT methods. The electronic density based vdW-DF2 and VV10 van der Waals density functionals have been tested, as well as an
Jörn Penger et al.
Applied and environmental microbiology, 78(21), 7596-7602 (2012-08-21)
In natural environments methane is usually produced by aceticlastic and hydrogenotrophic methanogenic archaea. However, some methanogens can use C(1) compounds such as methanol as the substrate. To determine the contributions of individual substrates to methane production, the stable-isotope values of
Enrique M Cabaleiro-Lago et al.
The Journal of chemical physics, 128(19), 194311-194311 (2008-05-27)
The characteristics of the interaction between phenol and acetonitrile, methyl fluoride and methyl chloride were studied. The most stable structures for clusters containing one or two CH3X molecules and one phenol moiety were located by means of ab initio and
Anne Daebeler et al.
PloS one, 8(1), e53656-e53656 (2013-01-24)
The metabolic pathways of methane formation vary with environmental conditions, but whether this can also be linked to changes in the active archaeal community structure remains uncertain. Here, we show that the suppression of aceticlastic methanogenesis by methyl fluoride (CH(3)F)
Jongwook Choi et al.
Science (New York, N.Y.), 332(6037), 1545-1548 (2011-06-28)
Carbon-fluorine bonds are the strongest known single bonds to carbon and as a consequence can prove very hard to cleave. Alhough vinyl and aryl C-F bonds can undergo oxidative addition to transition metal complexes, this reaction has appeared inoperable with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service