518751
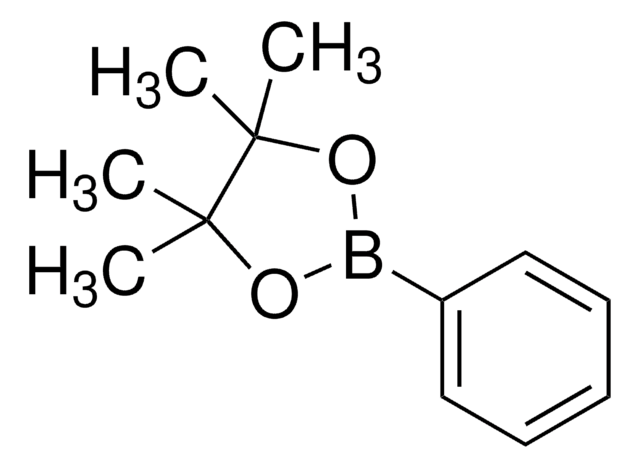
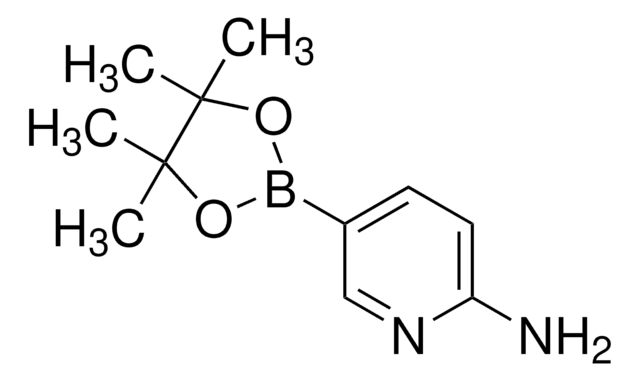
4-Aminophenylboronic acid pinacol ester
97%
Synonym(s):
2-(4-Aminophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)aniline, 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)benzeneamine, 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenylamine, 4-Aminophenylboronic acid, pinacol cyclic ester
About This Item
Recommended Products
Quality Level
assay
97%
mp
165-169 °C (lit.)
SMILES string
CC1(C)OB(OC1(C)C)c2ccc(N)cc2
InChI
1S/C12H18BNO2/c1-11(2)12(3,4)16-13(15-11)9-5-7-10(14)8-6-9/h5-8H,14H2,1-4H3
InChI key
ZANPJXNYBVVNSD-UHFFFAOYSA-N
Application
- The preparation of substituted 3-phenyl-4H-1-benzopyran-4-ones by reacting with iodochromones via Pd catalyzed Suzuki-Miyaura cross-coupling reaction.
- Mercury(II) detection by fluorometry with new fluorogenic indicators based on through-bond energy transfer from pentaquinone to rhodamine.
- Rhodium-catalyzed amination reactions.
- Palladium-catalyzed Suzuki cross-coupling to synthesize potential antitubercular and antimicrobial compounds.
It can also be used to prepare:
- Hexaphenylbenzene derivatives as a potential bioprobe and multichannel keypad system.
- Pyromellitic diimide-based polymer as matrix for solution-processable n-channel field-effect transistors.
- Alternating copolymers of oligoarylenes and naphthalene bisimides as low band-gap semiconductors with electrochemical and spectroelectrochemical behavior.
- γ-secretase modulators in the treatment of amyloid β formation.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service