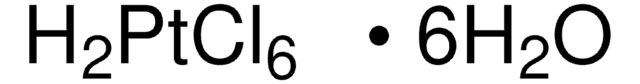520896
Chloroplatinic acid hydrate
≥99.9% trace metals basis
Synonym(s):
Hexachloroplatinic(IV) acid hydrate, Hydrogen hexachloroplatinate(IV) hydrate, Platinic chloride hydrate
Select a Size
Select a Size
About This Item
Recommended Products
assay
≥99.9% trace metals basis
composition
Pt, 37-40%
mp
60 °C (lit.)
density
2.43 g/mL at 25 °C (lit.)
SMILES string
O.Cl.Cl.Cl[Pt](Cl)(Cl)Cl
InChI
1S/6ClH.H2O.Pt/h6*1H;1H2;/q;;;;;;;+4/p-4
InChI key
SLIOYUPLNYLSSR-UHFFFAOYSA-J
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- in the determination of potassium, by selective precipitation as potassium chloroplatinate.
- in the purification of platinum.
- In catalysis during the addition of silicon hydrides to olefinic double bonds and in platinum-catalyzed hydrosilylation of alkynes.
Storage and Stability
2. Recommended for one time use to prevent water absorption and degradation. If necessary, once opened, repack and seal under dry inert gas.
3. Store with care. Prevent from light. Always store in well-sealed containers in a dry inert atmosphere. Recommended to store containers in a secondary desiccator with desiccant in a dry inert atmosphere.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Resp. Sens. 1 - Skin Corr. 1B - Skin Sens. 1
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service