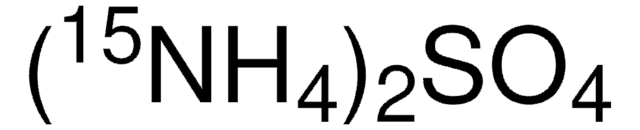544884
Iron(III) oxide
nanopowder, <50 nm particle size (BET)
Synonym(s):
Ferric oxide
Select a Size
Select a Size
About This Item
Recommended Products
description
crystalline (primarily γ)
Quality Level
form
nanopowder
surface area
50-245 m2/g
particle size
<50 nm (BET)
application(s)
battery manufacturing
SMILES string
O=[Fe]O[Fe]=O
InChI
1S/2Fe.3O
InChI key
JEIPFZHSYJVQDO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Features and Benefits
- High theoretical specific capacity
- Biocompatibility
- Ease of coating and modification
- Non-toxicity
Storage Class
13 - Non Combustible Solids
wgk_germany
nwg
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Innovation in dental restorative materials is driven by the need for biocompatible and natural-appearing restoration alternatives. Conventional dental materials like amalgam and composite resins have inherent disadvantages.
Magnetic nanoparticles have attracted tremendous attention due to their novel properties and their potential applications in magnetic recording, magnetic energy storage and biomedicine.
Graphene is a unique two-dimensional (2D) structure of monolayer carbon atoms packed into a dense honeycomb crystal that has attracted great interest due to its diverse and fascinating properties.
Professor Hui Mao explores the use of superparamagnetic iron oxide nanoparticles (INOPs) that offer an alternate contrast-enhancing mechanism.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service