656577
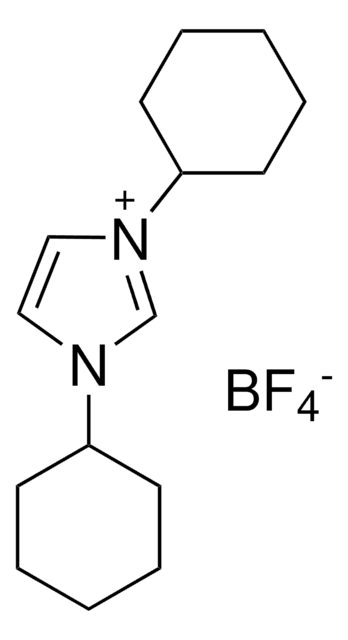
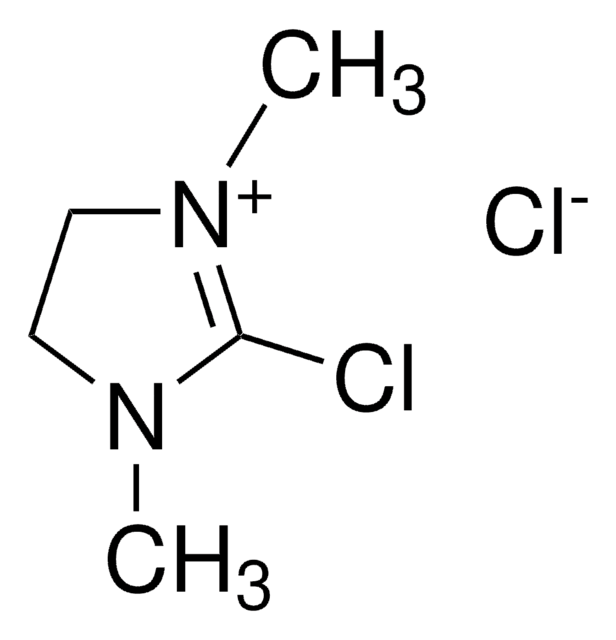
1,3-Diisopropylimidazolium chloride
97%
Synonym(s):
N,N′-(Isopropyl)imidazolium chloride
About This Item
Recommended Products
assay
97%
form
solid
reaction suitability
reagent type: catalyst
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
182-186 °C
greener alternative category
SMILES string
[Cl-].CC(C)n1cc[n+](c1)C(C)C
InChI
1S/C9H17N2.ClH/c1-8(2)10-5-6-11(7-10)9(3)4;/h5-9H,1-4H3;1H/q+1;/p-1
InChI key
DOFXKPAOJLLPII-UHFFFAOYSA-M
General description
Application
- 1,3-Diisopropylimidazole-2-thione by reacting with sulfur in the presence of potassium carbonate.
- (C9H7)NiCl(1,3-diisopropylimidazol-2- ylidene) by reacting with bis-indenyl nickel-(II).
- Ruthenium N-heterocyclic carbene complexes, which are efficient catalysts for the amidation of primary alcohols and amines.
Amide Synthesis from Alcohols and Amines by the Extrusion of Dihydrogen
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Acetylene chemistry's versatile applications from organic synthesis to bioorganic chemistry demand efficient synthesis methods.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service