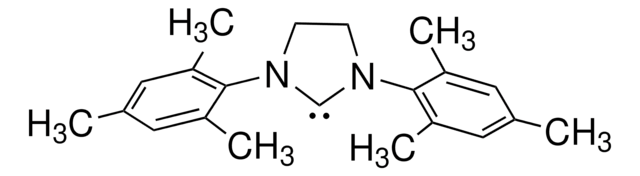
656623
1,3-Bis-(2,6-diisopropylphenyl)imidazolinium chloride
97%
Synonym(s):
N,N′-(2,6-Diisopropylphenyl)dihydroimidazolium chloride
About This Item
Recommended Products
Quality Level
assay
97%
form
solid
reaction suitability
reagent type: ligand
mp
289-293 °C (lit.)
SMILES string
[Cl-].CC(C)c1cccc(C(C)C)c1N2CC[N+](=C2)c3c(cccc3C(C)C)C(C)C
InChI
1S/C27H39N2.ClH/c1-18(2)22-11-9-12-23(19(3)4)26(22)28-15-16-29(17-28)27-24(20(5)6)13-10-14-25(27)21(7)8;/h9-14,17-21H,15-16H2,1-8H3;1H/q+1;/p-1
InChI key
LWPXTYZKAWSRIP-UHFFFAOYSA-M
Related Categories
Application
related product
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Metal complex-catalyzed cross-coupling reactions of unactivated substrates introduce diverse phosphine ligands in chemical marketplace.
A wide range of NHC ligands are commonly available which exhibit high activities.
The Hazari group has developed an improved palladium precatalyst scaffold for a wide range of cross-coupling reactions
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service









![Chloro[1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene]copper(I)](/deepweb/assets/sigmaaldrich/product/structures/199/763/44637b2e-b87c-42a3-abc3-3985b6cd7d5d/640/44637b2e-b87c-42a3-abc3-3985b6cd7d5d.png)