673986
2,6-Bis[(3aR,8aS)-(+)-8H-indeno[1,2-d]oxazolin-2-yl)pyridine
≥94%
Synonym(s):
(3aR,3′aR,8aS,8′aS)-2,2′-(2,6-Pyridinediyl)bis[3a,8a-dihydro-8H-indeno[1,2-d]oxazole, (3aR,8aS)-in-pybox
About This Item
Recommended Products
assay
≥94%
form
solid
optical activity
[α]20/D +386°, c = 1 in methylene chloride(lit.)
mp
284 °C (dec.)
functional group
ether
SMILES string
C1[C@@H]2OC(=N[C@@H]2c3ccccc13)c4cccc(n4)C5=N[C@H]6[C@H](Cc7ccccc67)O5
InChI
1S/C25H19N3O2/c1-3-8-16-14(6-1)12-20-22(16)27-24(29-20)18-10-5-11-19(26-18)25-28-23-17-9-4-2-7-15(17)13-21(23)30-25/h1-11,20-23H,12-13H2/t20-,21-,22+,23+/m0/s1
InChI key
BZSJUFJXCHHRHW-MYDTUXCISA-N
Application
- [3+2] cycloaddition of N-tosyl aziridines with electron-rich alkenes via selective carbon-carbon bond cleavage
- Enantioselective Mukaiyama-aldol reactions
- Enantioselective carbonyl-ene reactions
- Cu(I)-catalyzed asymmetric alkynylation of imino esters with terminal alkynes
- Asymmetric three-component coupling reactions catalyzed by a Cu/pybox complex
- Enantioselective Diels-Alder reaction
- Ni-catalyzed cross-coupling reactions
hcodes
Hazard Classifications
Aquatic Chronic 4
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The PYBOX ligand, consisting of a pyridine ring flanked by two oxazoline groups, was introduced in 1989 by Nishiyama.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
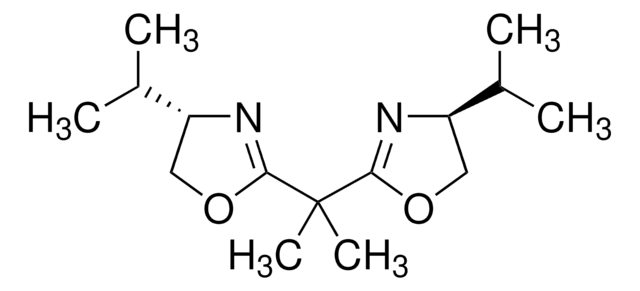
Contact Technical Service![2,6-Bis[(4S)-(−)-isopropyl-2-oxazolin-2-yl]pyridine 99%](/deepweb/assets/sigmaaldrich/product/structures/452/550/7e22a7c6-e84a-4741-af9a-e40f05d8061c/640/7e22a7c6-e84a-4741-af9a-e40f05d8061c.png)
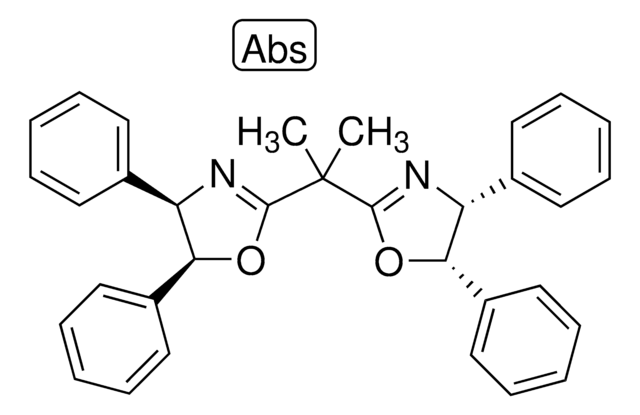
![2,6-Bis[(4S)-4-phenyl-2-oxazolinyl]pyridine 98%](/deepweb/assets/sigmaaldrich/product/structures/372/262/fb5c79fe-8277-48b0-a73e-4124c7c2c41c/640/fb5c79fe-8277-48b0-a73e-4124c7c2c41c.png)
![2,6-Bis[(3aS,8aR)-3a,8a-dihydro-8H-indeno[1,2-d]oxazolin-2-yl]pyridine](/deepweb/assets/sigmaaldrich/product/structures/302/311/68f9e406-0638-40fd-aafc-e36fe72b7ea8/640/68f9e406-0638-40fd-aafc-e36fe72b7ea8.png)
![(−)-2,2′-Isopropylidenebis[(4S)-4-phenyl-2-oxazoline] 97%](/deepweb/assets/sigmaaldrich/product/structures/297/720/a29f61c3-34e4-410c-acdd-241699b80af3/640/a29f61c3-34e4-410c-acdd-241699b80af3.png)
![2,6-Bis[(4R)-4-phenyl-2-oxazolinyl]pyridine 98%](/deepweb/assets/sigmaaldrich/product/structures/888/428/3be8313a-627a-4281-be35-306cc5da562a/640/3be8313a-627a-4281-be35-306cc5da562a.png)
![2,6-Bis[(4R)-(+)-isopropyl-2-oxazolin-2-yl]pyridine 99%](/deepweb/assets/sigmaaldrich/product/structures/349/609/8673c46e-368a-47a6-a9bd-52bbe13d490a/640/8673c46e-368a-47a6-a9bd-52bbe13d490a.png)
![2,2′-Isopropylidenebis[(4S)-4-tert-butyl-2-oxazoline] 99%](/deepweb/assets/sigmaaldrich/product/structures/334/357/19788a81-5365-46fd-978b-6b98382b1117/640/19788a81-5365-46fd-978b-6b98382b1117.png)
![[3aR-[2(3′aR*,8′aS*),3′aβ,8′aβ]]-(+)-2,2′-Methylenebis[3a,8a-dihydro-8H-indeno[1,2-d]oxazole] 98%](/deepweb/assets/sigmaaldrich/product/structures/134/031/294d2464-1571-4514-8e4c-c0cda1c1df7b/640/294d2464-1571-4514-8e4c-c0cda1c1df7b.png)
![2,2′-Bis[(4S)-4-benzyl-2-oxazoline] 98%](/deepweb/assets/sigmaaldrich/product/structures/139/783/42da3c77-52af-401b-8525-35d96415e284/640/42da3c77-52af-401b-8525-35d96415e284.png)
![2,2′-Methylenebis[(4S)-4-phenyl-2-oxazoline] 97%](/deepweb/assets/sigmaaldrich/product/structures/255/350/4403d4f8-c973-4da7-a5b6-2e93d1eacb10/640/4403d4f8-c973-4da7-a5b6-2e93d1eacb10.png)
![(4S)-(+)-Phenyl-α-[(4S)-phenyloxazolidin-2-ylidene]-2-oxazoline-2-acetonitrile 97%](/deepweb/assets/sigmaaldrich/product/structures/117/759/d4e6e882-8577-4dcf-84fe-506633ae811a/640/d4e6e882-8577-4dcf-84fe-506633ae811a.png)