678023
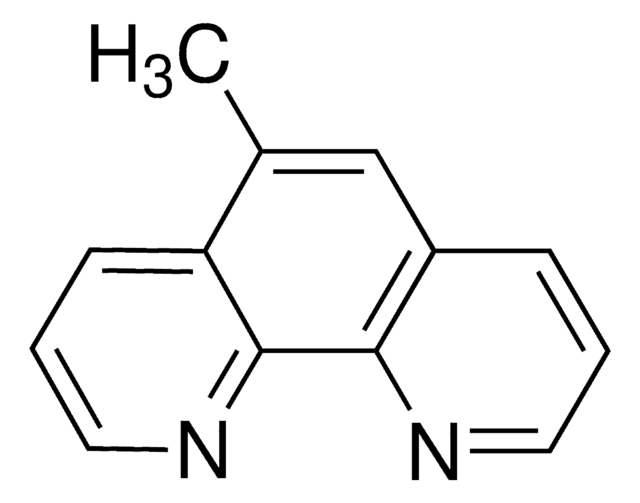
4,7-Dimethoxy-1,10-phenanthroline
97%
Synonym(s):
4,7-Dimethoxy-1,10-phenanthrolin
About This Item
Recommended Products
Quality Level
assay
97%
form
solid
reaction suitability
reagent type: ligand
mp
197-212 °C
SMILES string
COc1ccnc2c1ccc3c(OC)ccnc23
InChI
1S/C14H12N2O2/c1-17-11-5-7-15-13-9(11)3-4-10-12(18-2)6-8-16-14(10)13/h3-8H,1-2H3
InChI key
ZPGVCQYKXIQWTP-UHFFFAOYSA-N
General description
Application
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Eye Dam. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service










![5,8-Dimethyldibenzo[b,j][1,10]phenanthroline-6,7-diol](/deepweb/assets/sigmaaldrich/product/structures/369/172/ecac2dbe-a8f3-4161-aabb-18c93281f0e7/640/ecac2dbe-a8f3-4161-aabb-18c93281f0e7.png)