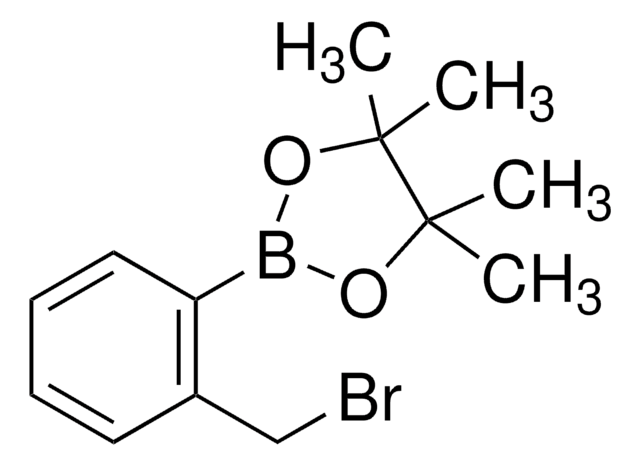
679453
2-(Bromomethyl)phenylboronic acid
Synonym(s):
α-Bromo-o-tolueneboronic acid, o-Boronobenzyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C7H8BBrO2
CAS Number:
Molecular Weight:
214.85
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
mp
158-162 °C
Quality Level
functional group
bromo
storage temp.
2-8°C
SMILES string
OB(O)c1ccccc1CBr
InChI
1S/C7H8BBrO2/c9-5-6-3-1-2-4-7(6)8(10)11/h1-4,10-11H,5H2
InChI key
MYVJCOQGXCONPE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
May contain varying amounts of anhydride.
Application
2-(Bromomethyl)phenylboronic acid can be used as a reactant to synthesize:
- Boronic acid-functionalized benzyl viologen (o-BBV) from 4,4′-bipyridine. o-BBV finds its application as a chemosensor to sense glucose in aqueous water.
- 2-(azidomethyl)phenylboronic acid, which is further employed in the preparation of isoquinoline derivatives.
- Aryl boronic acid derivatives as fluorescent probe for hydrogen peroxide detection.
Reactant involved in:
- Studies of carbon-boron bond cleavage of phenylboronate-pendant cyclen
- Development of a sensory system to detect glucose or other monosaccharides and hydroxycarboxylates
- Synthesis of boronated triaryl and tetraaryl phosphonium salts used in cytotoxicity studies
- Colorimetry and fluorometry mercury determination with chemosensors composed of rhodamine and boronic acid groups
- Synthesis of imidazolium-containing boronic acids used as fluoride ion sensors
- Reactions with adenine for molecules with antiinflammatory antitumor activities
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A highly sensitive water-soluble system to sense glucose in aqueous solution
Feng L, et al.
Organic & Biomolecular Chemistry, 9(8), 2938-2942 (2011)
A simple boronic acid-based fluorescent probe for selective detection of hydrogen peroxide in solutions and living cells
Han J, et al.
Bioorganic Chemistry, 81(7), 362-366 (2018)
2-(Azidomethyl) phenylboronic acid in the synthesis of isoquinoline derivatives
Ganina OG, et al.
Russian Chemical Bulletin, 54(7), 1606-1611 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service