690481
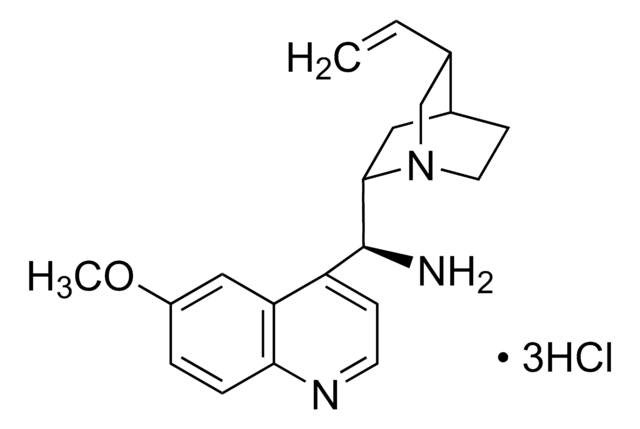
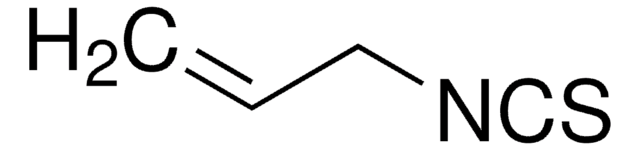
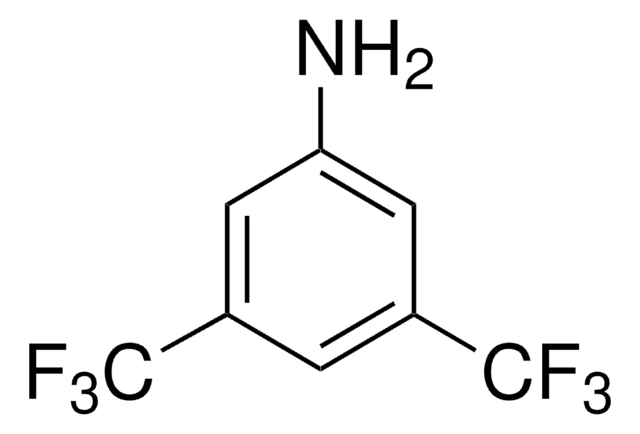
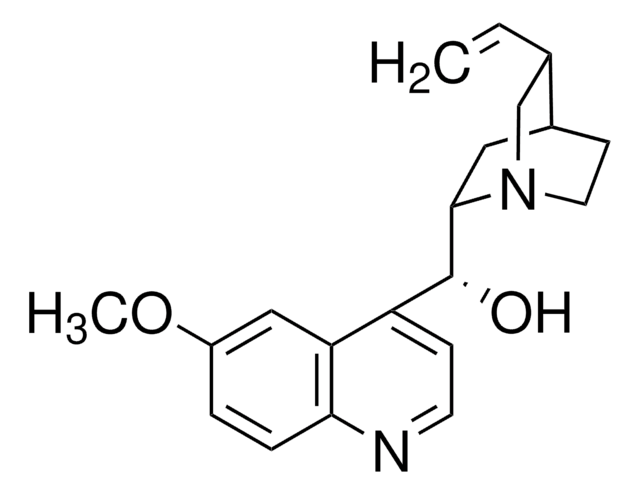
N-[3,5-Bis(trifluoromethyl)phenyl]-N′-[(8a,9S)-6′-methoxy-9-cinchonanyl]thiourea
90%
Synonym(s):
1-[3,5-Bis(trifluoromethyl)phenyl)-3-{(S)(6-methoxy-4-quinolinyl)-[(2S,4S,5R)-5-vinyl-1-aza-bicyclo[2.2.2]oct-2-yl]methyl}thiourea, epi-N-Quinyl-N′-bis(3,5-trifluoromethyl)phenylthiourea
About This Item
Recommended Products
Quality Level
assay
≥89.0%
90%
form
lumps
functional group
amine
fluoro
thiourea
SMILES string
COc1ccc2nccc([C@H](NC(=S)Nc3cc(cc(c3)C(F)(F)F)C(F)(F)F)C4CC5CCN4C[C@@H]5C=C)c2c1
InChI
1S/C29H28F6N4OS/c1-3-16-15-39-9-7-17(16)10-25(39)26(22-6-8-36-24-5-4-21(40-2)14-23(22)24)38-27(41)37-20-12-18(28(30,31)32)11-19(13-20)29(33,34)35/h3-6,8,11-14,16-17,25-26H,1,7,9-10,15H2,2H3,(H2,37,38,41)/t16-,17-,25-,26-/m0/s1
InChI key
IQMKPBFOEWWDIQ-FRSFCCSCSA-N
Related Categories
Application
- Stereoselective diaryl(nitro)butanone via enantioselective Michael addition of nitromethane to chalcones.
- Enantioselective β-amino acids via asymmetric Mannich reaction of malonates with aryl and alkyl imines.
- The synthesis of 3-indolylmethanamines by the reaction of indoles with imines via asymmetric Friedel-Crafts reaction.
- The enantioselective conjugate addition of active methylene compounds to enones to obtain the corresponding addition products.
Packaging
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![1-[3,5-bis(trifluoromethyl)phenyl]-3-[(1R,2R)-(-)-2-(dimethylamino)cyclohexyl]thiourea AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/236/021/d944889d-2233-4700-9f2c-caa3652d0124/640/d944889d-2233-4700-9f2c-caa3652d0124.png)
![N-[(1R,2R)-2-(1-Piperidinyl)cyclohexyl]-N′-[4-(trifluoromethyl)phenyl]squaramide 95%](/deepweb/assets/sigmaaldrich/product/structures/238/480/7149c9c0-8769-418a-a96c-77c15dd50cd0/640/7149c9c0-8769-418a-a96c-77c15dd50cd0.png)
![N-[3,5-Bis(trifluoromethyl)phenyl]-N′-[(8a,9S)-10,11-dihydro-6′-methoxy-9-cinchonanyl]thiourea 90%](/deepweb/assets/sigmaaldrich/product/structures/321/413/b3c0b73b-9ee7-4dea-b9cd-795beb4cdbfa/640/b3c0b73b-9ee7-4dea-b9cd-795beb4cdbfa.png)
![1-[3,5-Bis(trifluoromethyl)phenyl]-3-[(1R,2R)-(−)-2-(dimethylamino)cyclohexyl]thiourea](/deepweb/assets/sigmaaldrich/product/structures/384/772/d336462c-f438-446d-be0c-4064705213cc/640/d336462c-f438-446d-be0c-4064705213cc.png)
![(S)-2-[[3,5-Bis(trifluoromethyl)phenyl]thioureido]-N-benzyl-N,3,3-trimethylbutanamide 97%](/deepweb/assets/sigmaaldrich/product/structures/373/888/118b46f2-6c2e-4a87-8266-c4dbcd5db51f/640/118b46f2-6c2e-4a87-8266-c4dbcd5db51f.png)