All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C82H86Cl4N8O2S5
Molecular Weight:
1517.75
UNSPSC Code:
12352101
NACRES:
NA.23
Recommended Products
Related Categories
General description
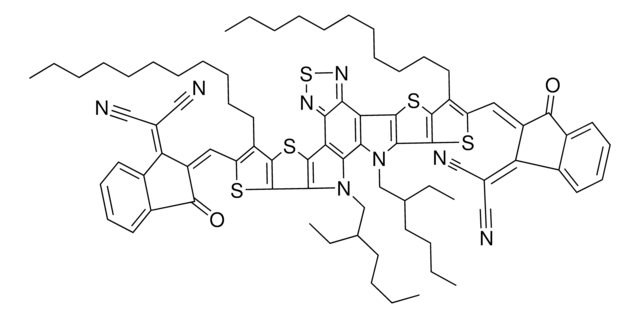
Synonym: 2,2′-((2Z,2′Z)-((12,13-bis(2-ethylhexyl)-3,9-diundecyl-12,13-dihydro-[1,2,5]thiadiazolo[3,4-e]thieno[2",3":4′,5′]-thieno[2′,3′:4,5]pyrrolo[3,2-g]thieno[2′,3′:4,5]thieno[3,2-b]indole-2,10-diyl)bis(methanylylidene))bis(5,6-dichloro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile
Application
BTP-4Cl is a high performance, low bandgap, non-fullerene accpetor (NFA). It is the chlorinated derivative of the fused benzothiadiazole-based NFA, Y6. It offer high power conversion efficiency (PCE) when pairing with p-type polymer PM6. A 9 mm2 single junction device of this blend provided a PCE of 16.5% at thickness of ~ 100nm, and provided a PCE of >13% at a thickness of ~300 nm (for the active layer). An impressive PCE of >15% was reached for this blend with a 1 cm2 active layer. In general, Y7 showed improved performances over Y6, mainly due to its highest photoluminescence, lower non-radiative energy loss, and a higher Voc of 0.867 V vs 0.834 V for Y6.
Y7 is a highly conjugated organic semiconductor, electron deficient due to its structure and hence suitable for use as a n-type non-fullerene electron acceptor(NFA) in OPV devices. It has an absorption range that extends to the near infrared (NIR) and has demonstrated a power conversion efficiency (PCE) up to15.7% with PBDB-T-2F (PM6) as the polymer donor.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yong Cui et al.
Nature communications, 10(1), 2515-2515 (2019-06-09)
Broadening the optical absorption of organic photovoltaic (OPV) materials by enhancing the intramolecular push-pull effect is a general and effective method to improve the power conversion efficiencies of OPV cells. However, in terms of the electron acceptors, the most common
Related Content
Organic electronics utilizes organic conductors and semiconductors for applications in organic photovoltaics, organic light-emitting diodes, and organic field-effect transistors.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service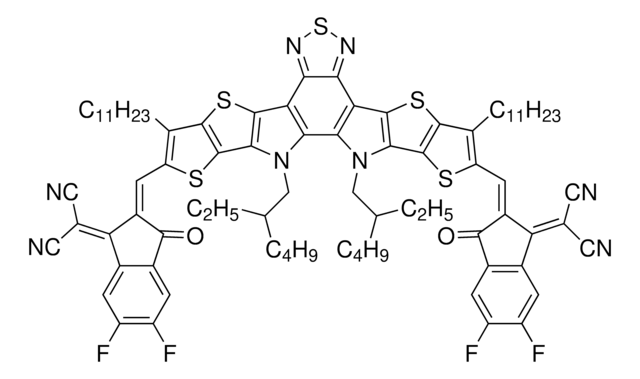
![[6,6]-Phenyl C71 butyric acid methyl ester, mixture of isomers 99%](/deepweb/assets/sigmaaldrich/product/structures/716/624/9fb9f2f0-ae99-429f-8d3a-b12267976a4d/640/9fb9f2f0-ae99-429f-8d3a-b12267976a4d.png)
