930423
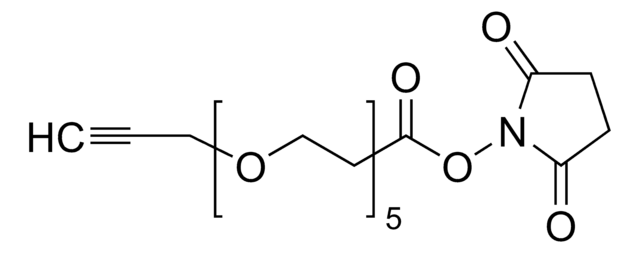
CP-alkyne
≥95%
Synonym(s):
2,4,6-Trimethyl-1-(methyl((pent-4-yn-1-yloxy)carbonyl)amino)pyridin-1-ium tetrafluoroborate
About This Item
Recommended Products
description
Application: Chemoproteomics
Quality Level
assay
≥95%
form
liquid
storage temp.
−20°C
SMILES string
CC1=CC(C)=[N+](N(C(OCCCC#C)=O)C)C(C)=C1.F[B-](F)(F)F
InChI key
JEYOQLIMTNLUGZ-UHFFFAOYSA-N
Application
Other Notes
2. A quantitative thiol reactivity profiling platform to analyze redox and electrophile reactive cysteine proteomes
3. Ethynylation of Cysteine Residues: From Peptides to Proteins in Vitro and in Living Cells
4. A Chemoproteomic Platform To Assess Bioactivation Potential of Drugs
5. Inhibition of Zinc-Dependent Histone Deacetylases with a Chemically Triggered Electrophile
6. Reversibility of Covalent Electrophile-Protein Adducts and Chemical Toxicity
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service