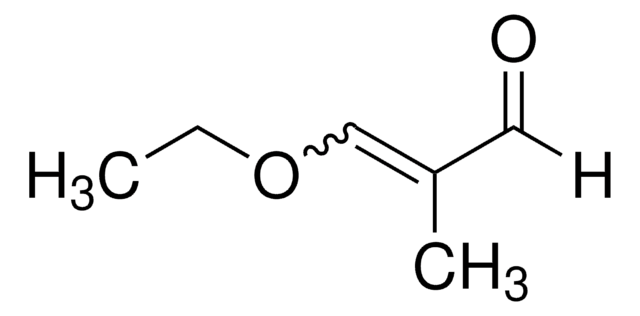
A24001
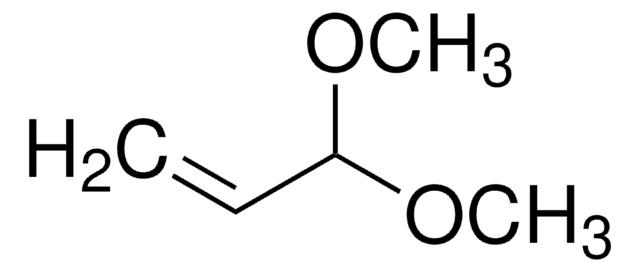
Acrolein diethyl acetal
96%
Synonym(s):
3,3-Diethoxy-1-propene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH2=CHCH(OCH2CH3)2
CAS Number:
Molecular Weight:
130.18
Beilstein/REAXYS Number:
1701567
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
96%
form
liquid
refractive index
n20/D 1.398 (lit.)
bp
125 °C (lit.)
density
0.854 g/mL at 25 °C (lit.)
SMILES string
CCOC(OCC)C=C
InChI
1S/C7H14O2/c1-4-7(8-5-2)9-6-3/h4,7H,1,5-6H2,2-3H3
InChI key
MCIPQLOKVXSHTD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Acrolein diethyl acetal is widely used to carry out chemoselective Heck arylation to synthesize either 3-arylpropanoate esters or cinnamaldehyde derivatives.
It can also be used as one of the precursors to synthesize natural products like (−)-(Z)-Deoxypukalide, (−)-Laulimalide, botryodiplodin, neolaulimalide and isolaulimalide.
It can also be used as one of the precursors to synthesize natural products like (−)-(Z)-Deoxypukalide, (−)-Laulimalide, botryodiplodin, neolaulimalide and isolaulimalide.
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
59.0 °F - closed cup
flash_point_c
15 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of (?)-and (−)-botryodiplodin using stereoselective radical cyclizations of acyclic esters and acetals.
Nouguier R et al.
Tetrahedron Asymmetry, 14(19), 3005-3018 (2003)
Total Synthesis of Microtubule-Stabilizing Agent (-)-Laulimalide1.
Ghosh AK et al.
The Journal of Organic Chemistry, 66(26), 8973-8982 (2001)
Stefano Parisotto et al.
Organic & biomolecular chemistry, 15(4), 884-893 (2017-01-04)
As part of our ongoing work on the synthesis of a new class of plant hormones named Strigolactones (SLs) and their analogues, we became interested in tracing bioactive molecules with red emitting BODIPY fluorophores in order to unravel signaling and
Mohammad Saidur Rhaman et al.
Plant & cell physiology, 61(5), 967-977 (2020-03-08)
Myrosinase (β-thioglucoside glucohydrolase, enzyme nomenclature, EC 3.2.1.147, TGG) is a highly abundant protein in Arabidopsis guard cells, of which TGG1 and TGG2 function redundantly in abscisic acid (ABA)- and methyl jasmonate-induced stomatal closure. Reactive carbonyl species (RCS) are α,β-unsaturated aldehydes
An efficient palladium-catalyzed synthesis of cinnamaldehydes from acrolein diethyl acetal and aryl iodides and bromides.
Battistuzzi G, et al.
Organic Letters, 5(5), 777-780 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service