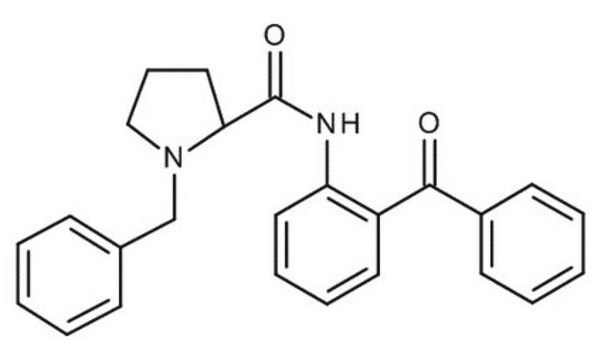
A41208
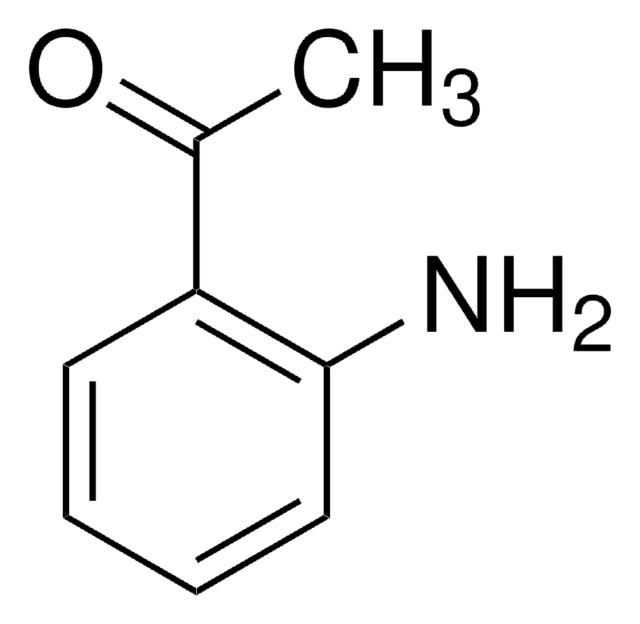
2-Aminobenzophenone
98%
Synonym(s):
2-Aminophenyl phenyl ketone, 2-Benzoylaniline
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
H2NC6H4C(O)C6H5
CAS Number:
Molecular Weight:
197.23
Beilstein/REAXYS Number:
743425
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
crystals
mp
103-107 °C (lit.)
SMILES string
Nc1ccccc1C(=O)c2ccccc2
InChI
1S/C13H11NO/c14-12-9-5-4-8-11(12)13(15)10-6-2-1-3-7-10/h1-9H,14H2
InChI key
MAOBFOXLCJIFLV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Luigi Aurelio et al.
Journal of medicinal chemistry, 53(18), 6550-6559 (2010-09-03)
2-Amino-3-benzoylthiophenes (2A3BTs) have been widely reported to act as allosteric enhancers (AEs) at the A(1) adenosine receptor (A(1)AR). Herein we describe the synthesis of a series of 1-aminoindeno[1,2-c]thiophen-8-ones and a series of (2-aminoindeno[2,1-b]thiophen-3-yl)(phenyl)methanones as conformationally rigid analogues of the 2A3BTs.
Hee J Park et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 102, 172-179 (2017-03-11)
A doubly enteric-coated multiple-unit tablet (DET) of bisacodyl (BD) was formulated to selectively deliver the stimulant laxative to the large intestine. Solubilized BD in surfactants was adsorbed into the porous carrier and primarily coated with different combinations of pH-sensitive polymers
Rasa Keruckiene et al.
Beilstein journal of organic chemistry, 16, 1142-1153 (2020-06-20)
Three compounds, bearing a quinazoline unit as the acceptor core and carbazole, dimethyldihydroacridine, or phenothiazine donor moieties, were designed and synthesized in two steps including a facile copper-catalyzed cyclization and a nucleophilic aromatic substitution reaction. The photophysical properties of the
Minakshi Ghate et al.
Luminescence : the journal of biological and chemical luminescence, 33(6), 999-1009 (2018-06-01)
This paper reports the synthesis and characterization of 2-(4-ethoxyphenyl)-4-phenyl quinoline (OEt-DPQ) organic phosphor using an acid-catalyzed Friedlander reaction and the preparation of blended thin films by molecularly doping OEt-DPQ in poly(methyl methacrylate) (PMMA) at different wt%. The molecular structure of
Víctor González-Ruiz et al.
PloS one, 9(5), e95998-e95998 (2014-05-17)
Topoisomerase 1 inhibition is an important strategy in targeted cancer chemotherapy. The drugs currently in use acting on this enzyme belong to the family of the camptothecins, and suffer severe limitations because of their low stability, which is associated with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service