All Photos(2)
About This Item
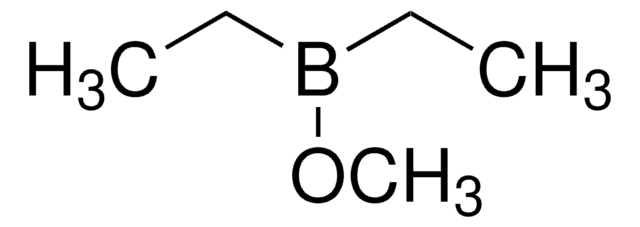
Linear Formula:
CH3COOC(CH3)3
CAS Number:
Molecular Weight:
116.16
Beilstein/REAXYS Number:
1699506
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥99%
form
liquid
refractive index
n20/D 1.386 (lit.)
bp
97-98 °C (lit.)
density
0.866 g/mL at 20 °C (lit.)
SMILES string
CC(=O)OC(C)(C)C
InChI
1S/C6H12O2/c1-5(7)8-6(2,3)4/h1-4H3
InChI key
WMOVHXAZOJBABW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
tert-Butyl acetate can be used to convert:
- Aromatic nitriles to the corresponding N-tert-butylamides catalyzed by sulfuric acid.
- S-tert-Butyl-L-cysteine hydrochloride to S-tert-butyl-L-cysteine tert-butyl ester hydrochloride.
- Lanthanide isopropoxides to lanthanide tert-butoxides in cyclohexane.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Inhalation - Flam. Liq. 2 - STOT SE 3
target_organs
Central nervous system, Respiratory system
supp_hazards
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
39.2 °F - closed cup
flash_point_c
4 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
tert-Butyl group as thiol protection in peptide synthesis.
Pastuszak J J and Chimiak A
The Journal of Organic Chemistry, 46(9), 1868-1873 (1981)
Preparation and some reactions of alkoxides of gadolinium and erbium.
Mehrotra R C and Batwara J M
Inorganic Chemistry, 9(11), 2505-2510 (1970)
Morten Jørgensen et al.
Journal of the American Chemical Society, 124(42), 12557-12565 (2002-10-17)
A catalytic amount of Pd(dba)(2) ligated by either carbene precursor N,N'-bis(2,6-diisopropylphenyl)-4,5-dihydroimidazolium (1) or P(t-Bu)(3) mediated the coupling of aryl halides and ester enolates to produce alpha-aryl esters in high yields at room temperature. The reaction was highly tolerant of functionalities
An efficient method for the conversion of aromatic and aliphatic nitriles to the corresponding N-tert-butyl amides: a modified Ritter reaction.
Reddy KL.
Tetrahedron Letters, 44(7), 1453-1455 (2003)
Wenjun Du et al.
Biomacromolecules, 9(10), 2826-2833 (2008-09-18)
Three hyperbranched fluoropolymers were synthesized and their micelles were constructed as potential (19)F MRI agents. A hyperbranched star-like core was first synthesized via atom transfer radical self-condensing vinyl (co)polymerization (ATR-SCVCP) of 4-chloromethyl styrene (CMS), lauryl acrylate (LA), and 1,1,1-tris(4'-(2''-bromoisobutyryloxy)phenyl)ethane (TBBPE).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service